Published online Aug 21, 2011. doi: 10.3748/wjg.v17.i31.3614
Revised: March 24, 2011
Accepted: April 3, 2011
Published online: August 21, 2011
AIM: To investigate colorectal uptake of solid lipid nanoparticles (SLNs) in mice receiving different doses of 1,2-dimethylhydrazine (DMH) using magnetic resonance (MR) and laser-scanning confocal fluorescence microscope (LSCFM) imaging.
METHODS: Eight mice were sacrificed in a pilot study to establish the experimental protocol and to visualize colorectal uptake of SLNs in normal mice. Gadopentetate dimeglumine and fluorescein isothiocyanate (FITC)-loaded SLN (Gd-FITC-SLN) enemas were performed on mice receiving DMH for 10 wk (group 1, n = 9) or 16 wk (group 2, n = 7) and FITC-SLN enema was performed on 4 DMH-treated mice (group 3). Pre- and post-enema MR examinations were made to visualize the air-inflated distal colorectum. Histological and LSCFM examinations were performed to verify colorectal malignancy and to track the distribution of SLNs.
RESULTS: Homogeneous enhancement and dense fluorescence (FITC) deposition in colorectal wall were observed in normal mice and 1 DMH-treated mouse (group 1) on fluid attenuated inversion recovery (FLAIR) and LSCFM images, respectively. Heterogeneous mural enhancement was found in 6 mice (4 in group 1; 2 in group 2). No visible mural enhancement was observed in the other mice. LSCFM imaging revealed linear fluorescence deposition along the colorectal mucosa in all groups. Nine intraluminal masses and one prolapsed mass were detected by MR imaging with different enhancement modes and pathologies. Interstitial FITC deposition was identified where obvious enhancement was observed in FLAIR images. Bladder imaging agent accumulations were observed in 11 of 16 DMH-treated mice of groups 1 and 2.
CONCLUSION: There are significant differences in colorectal uptake and distribution of SLNs between normal and DMH-treated mice, which may provide a new mechanism of contrast for MR colonography.
- Citation: Wu T, Zheng WL, Zhang SZ, Sun JH, Yuan H. Bimodal visualization of colorectal uptake of nanoparticles in dimethylhydrazine-treated mice. World J Gastroenterol 2011; 17(31): 3614-3622
- URL: https://www.wjgnet.com/1007-9327/full/v17/i31/3614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i31.3614
Colorectal imaging examinations consist of double-contrast barium enema (DCBE), colonoscopy, computed tomography (CT) colonography and magnetic resonance (MR) colonography. The four methods have their advantages and disadvantages. Colonoscopy, however, is the dominant technique for colorectal examination due to its high diagnostic sensitivity and capability for immediate intervention and histological evaluation. Despite the merits and continuous technical innovation, colonoscopy, as an invasive test, is not well accepted in a screening setting and cannot be performed on certain conditions such as inflammatory or malignant bowel stenosis. MR colonography, introduced in the last decade, has demonstrated encouraging initial results in the detection of polyps greater than 1 cm in diameter. Unlike CT colonography, MR colonography is a “pure noninvasive” test because it is ion-free[1-3].
High contrast between the bowel wall and lumen, implemented by either “dark-lumen” or “bright-lumen” technique, is essential for successful MR colonography. The contrast mechanisms depend on the combination of ultrafast MR sequences and an appropriate rectal enema recipe[3-7]. In most cases, a low uptake of bowel contrast agents means low toxicity. To the best of our knowledge, no radiological research aiming at visualizing the uptake of bowel contrast agents has been described.
Recently, however, intestinal uptake of particulate matter in the micro- and nanometer range has been a hot topic in pharmacological research. Studies on oral delivery of insulin, vaccine and a set of hydrophobic drugs using various nano-vehicles are under way[8-13]. Solid lipid nanoparticles (SLN) are colloidal carriers for controlled drug delivery introduced after the development of emulsions, liposomes, polymer-based microparticles and nanoparticles. SLN combines the advantages of polymeric nanoparticles and oil/water fat emulsions for drug delivery, such as good tolerability, high oral bioavailability and feasibility, for large-scale production[14] .
The aim of this study is to exemplify the feasibility of using colorectal uptake of SLNs as an extra source of contrast in colonography. To model non-familial colorectal carcinoma (CRC) in rodents, 1,2-dimethylhydrazine (DMH), a specific colon carcinogen, was administered to the mice to produce CRC and impair the bowel wall. Colorectal uptake of SLNs in mice receiving different doses of DMH was investigated using MR and laser-scanning confocal fluorescence microscope (LSCFM) imaging.
Fluorescein isothiocyanate-labeled octadecylamine (ODA-FITC) and gadopentetate dimeglumine (Gd-DTPA) loaded SLNs (Gd-FITC-SLN) were synthesized by “solvent diffusion method in a nano-reactor system,” as described previously [15]. Briefly, Gd-DTPA (25 mg) and Tween 80 (18 mg) were dissolved in water (1 mL) to prepare the “aqueous phase”. The water in an oil mini-emulsion was obtained by mixing, stirring and ultrasonic treatment of the “aqueous phase” and the “oil phase,” which consisted of Span 80 (200 mg) and n-Hexane (10 mL). A mixture of 45 mg monostearin and 5 mg ODA-FITC, dissolved in 1 mL ethanol in a 60 °C water bath, was quickly dispersed into the mini-emulsion under mechanical agitating at 400 for 5 min. The dispersion was centrifuged for 15 min at 20 000 r/min to precipitate SLNs, which were subsequently washed twice with n-hexane and re-dispersed in Poloxamer 188. The resultant SLNs, dispersed to equal milligrams of manicol, were freeze-dried and kept away from light at 4 °C. Both Gd-DTPA and ODA-FITC were omitted to produce blank SLN; only one of the imaging agents, Gd-DTPA or ODA-FITC, was added to synthesize Gd-SLN or FITC-SLN. The physicochemical properties of the SLNs were characterized as documented previously [11,15].
All animal experiments were approved by the institutional animal care and use committee and performed in accordance with the committee’s regulations. The mice were deprived of food and allowed to drink 5% glucose saline 24 h before the examination to clean the gastrointestinal tract. An intra-peritoneal injection of pentobarbital (50 mg/kg body weight) was performed before any surgical manipulation. Eight male Kunming mice (22-25 g) were sacrificed in the pilot study. An operative procedure was established to limit enema within the distal colorectum.
MR pulse sequence (SE T2WI and FLAIR) and microscopic fluorescence imaging techniques were evaluated. The concentration of enema agents, including Gd-DTPA solution, Gd-SLN, FITC-SLN and Gd-FITC-SLN suspensions, were adjusted according to MR and fluorescence image findings. Qualified data from the pilot study were included into the study results.
Subcutaneous injection of DMH (20 mg/kg body weight) was performed wkly on 5-wk-old Kunming mice for 10 (n = 15) and 16 wk (n = 15) to induce colorectal tumors. Ten mice were excluded from the study due to DMH- and anesthesia-related mortality (n = 7) and operation failures (n = 3).
Gd-FITC-SLN (40 mg/mL) enema was performed on 9 mice receiving DMH for 10 wk (group 1) and 7 mice receiving DMH for 16 wk (group 2). FITC-SLN (40 mg/mL) enemas were performed on 4 mice (group 3) receiving DMH for 10 (n = 2) and 16 wk (n = 2).
After anesthesia, an abdominal incision was made into the peritoneal cavity, and the sigmoid colon was ligated. The peritoneal cavity was then closed by two layers of continuous sutures. Subsequently, the distal colorectum was slightly inflated by infusing about 0.3 mL room air via the anal orifice and gently ligating tissues around to prevent air leakage. Thus, the mouse was ready for the pre-enema MR examination. After the pre-enema MR test, an SLN enema was performed for 20 min by infusing 0.3-0.4 mL of the dispersion into the rectal lumen and ligating tissues around the anus. In-enema MR imaging was performed during the enema process. After the enema was performed and the anal ligate was removed, the enema agents were cleared by warm saline coloclysis. The distal colorectal lumen was then inflated by air again for the post-enema MR examinations, performed 25 and 60 min after the SLN enema was started. The mice were warmed by placing a hot water bag aside during the experiment.
Image acquisition was performed with a 1.5 T clinical MR device (Signa 1.5 T; GE Medical Systems, Milwaukee, Wis). A 5-cm custom-built coil was used for signal emission and reception. Animals were examined in the supine position. Transverse FLAIR MR images from sigmoid colon to the anus were acquired using the following parameters: repetition time, 2000 ms; echo time, 11.1 ms; inversion time, 750 ms; section thickness, 2 mm; intersection gap, 0 mm; field of view, 6-8 cm; matrix, 320 × 192; number of signals acquired, one. Transverse T2WI imaging (repetition time, 3860 ms; echo time, 106.0 ms) with the same section thickness and image size was also performed. Multi-planar FLAIR and T2WI imaging were performed continuously if colorectal masses had been detected in initial imaging.
The MR images of the colorectal wall and masses were at first interpreted in consensus by two radiologists with 20 and 10 years of experience, respectively. Colorectal masses were located by measuring the mass to anus distance. Then, quantitative analysis was performed based on the recommended procedure[16]. First, identical axial FLAIR slices before and after enema were selected for region of interest (ROI) definition. Second, a curved ROI encompassing the colorectal wall or an irregular ROI encompassing the intraluminal mass, a round ROI on the back or pelvic muscle and an oval ROI along the phase encoding direction encircling air were defined; the signal intensity (SI) values were recorded (Image J, version 1.38; National Institutes of Health, Bethesda, MD). Third, Third, the the SI difference–to-noise ratios (SDNRs) for the colorectal wall or tumors were calculated using the following formula: SDNR = (SIt-SIm)/SDN, where SIt is the mean SI value of target (the colorectal wall or intraluminal mass); SIm, the mean SI of the muscle; and SDN, the standard deviation of the background noise (air).
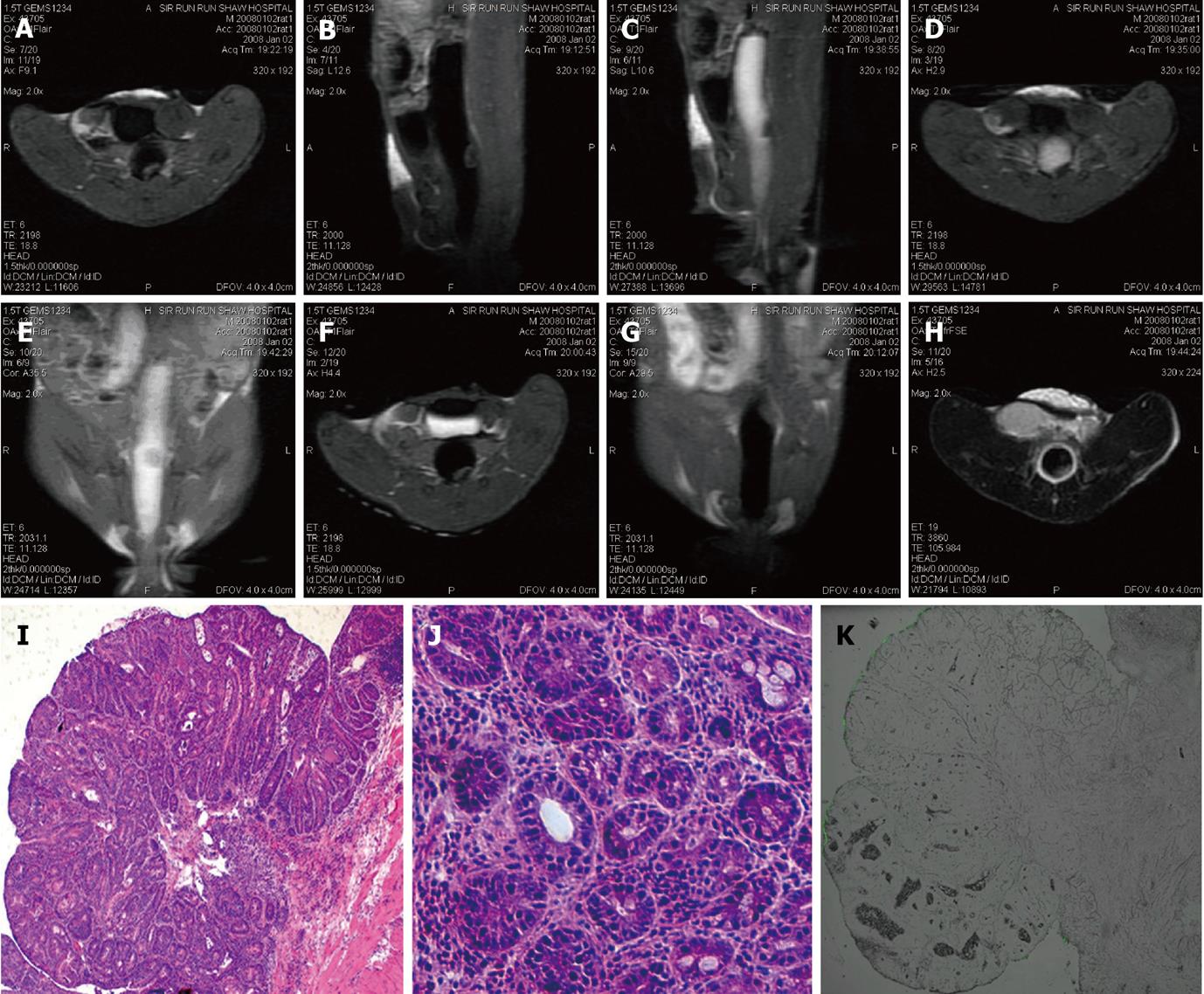
Animals were euthanized by an overdose of pentobarbital immediately after MR examination. The colorectum was harvested. The macroscopic morphology of the bowel as well as the location and size of the masses within the ligated distal colorectum were recorded. The colorectal wall and masses were then sampled, frozen with liquid nitrogen, and cut into 5-7 μm slices with a microtome for LSCFM (Leica TCS-SP5, Wetzlar, Germany) evaluation and HE slice preparation. Diamidino-phenyl-indole (DAPI 1:15 000 dilution, Sigma, St. Louis, MO, United States) staining was performed on slices of one normal mouse to visualize the nuclei of intestinal cells. FITC carried by SLNs was excited at 488 nm and detected at 500-535 nm wavelengths. DAPI was excited at 405 nm and detected at 430-550 nm. The remaining tissues were sampled and immerged in 10% buffered formalin to prepare the standard hematoxylin and eosin (HE)-stained slices.
SDNR data of colorectal wall, intraluminal mass size and other observations were expressed as means ± standard deviations. Statistical analysis was performed with software (SPSS for Windows, release 16.0; SPSS, Chicago). One-way analysis of variance with least significant difference tests was applied for multiple comparisons of pre- and post-enema SDNRs of the colorectal wall (groups 1-3) and SDNRs of intraluminal masses (groups 2 and 3). P value < 0.01 was considered a significant difference.
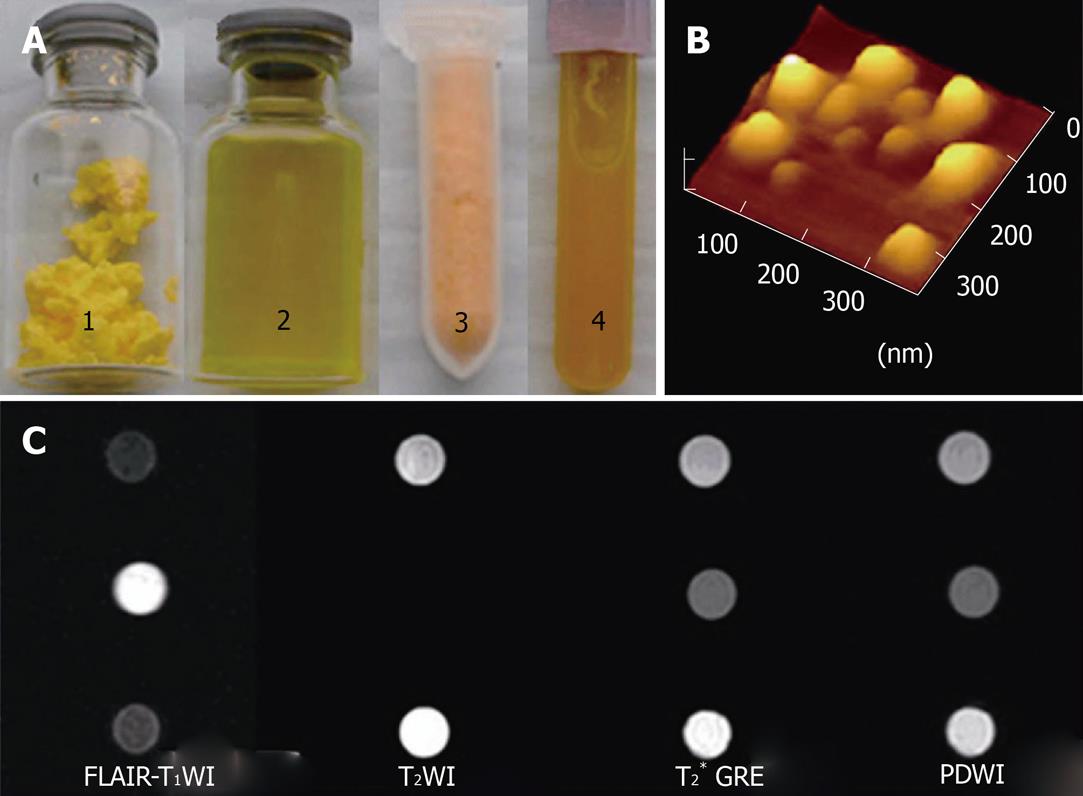
SLNs exhibited bimodal particle sizes ranging from 50 to 300 nm and zeta potentials ranging from - 29.3 ± 3.4 to - 39.1 ± 2.0 mV. The particle size increased slightly as ODA-FITC was loaded. Entrapment efficiency for Gd-DTPA in Gd-SLNs or in Gd-FITC-SLNs was 55.8% or 55.0%, respectively. Loading capacity of Gd-DTPA in Gd-SLNs or Gd-FITC-SLNs was about 50%. Hence, 40 mg Gd-FITC-SLN or Gd-SLN freeze-dried powder dispersed in 1 mL water, as used in the current study, contains about 10 mg Gd-DTPA and 20 mg Mannitol. MR images of SLN dispersions and pure water are shown in Figure 1.
Four mice were sacrificed to establish the experimental protocol. The colorectal wall was detectable on FLAIR (low-SI) and T2WI (iso- to high-SI) images. However, bowel layers could not be differentiated. While LSCFM (Leica TCS-SP5, Germany) provided fine fluorescent images, the fluorescence microscope (Zeiss Axioskop 2, Carl Zeiss, Marburg, Germany) seemed applicable in tracking the distribution of FITC-loaded SLNs.
Homogeneous mural enhancement on post-enema FLAIR images was observed after Gd-SLN (n = 1) and Gd-FITC-SLN (n = 2, 20/22 slices) retention enema. Dense FITC deposition was observed in fluorescence imaging after FITC-SLN (n = 1) and Gd-FITC-SLN enema (Figure 2). No positive MR or fluorescence image finding was observed after Gd-DTPA solution (n = 1) enema.
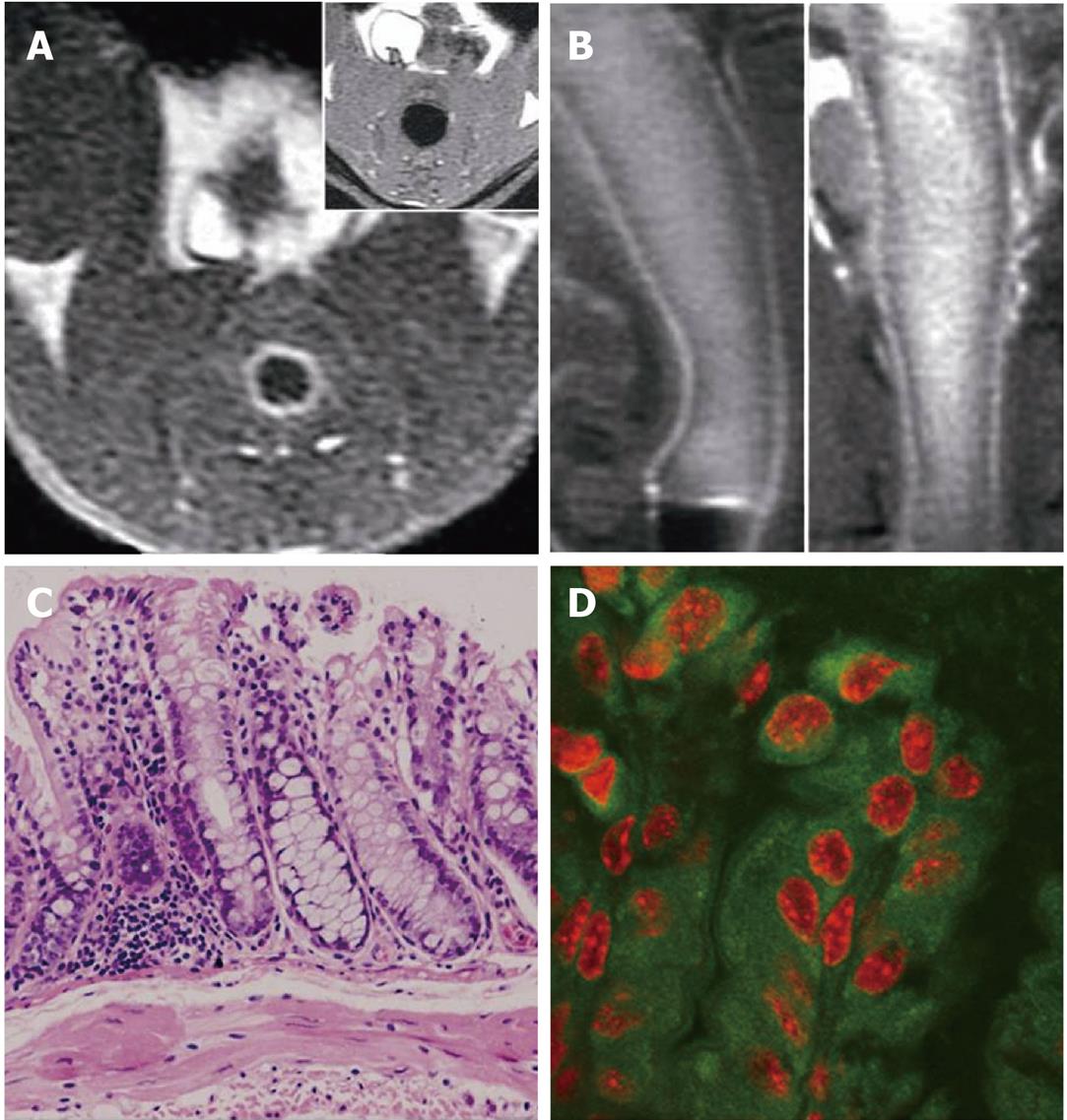
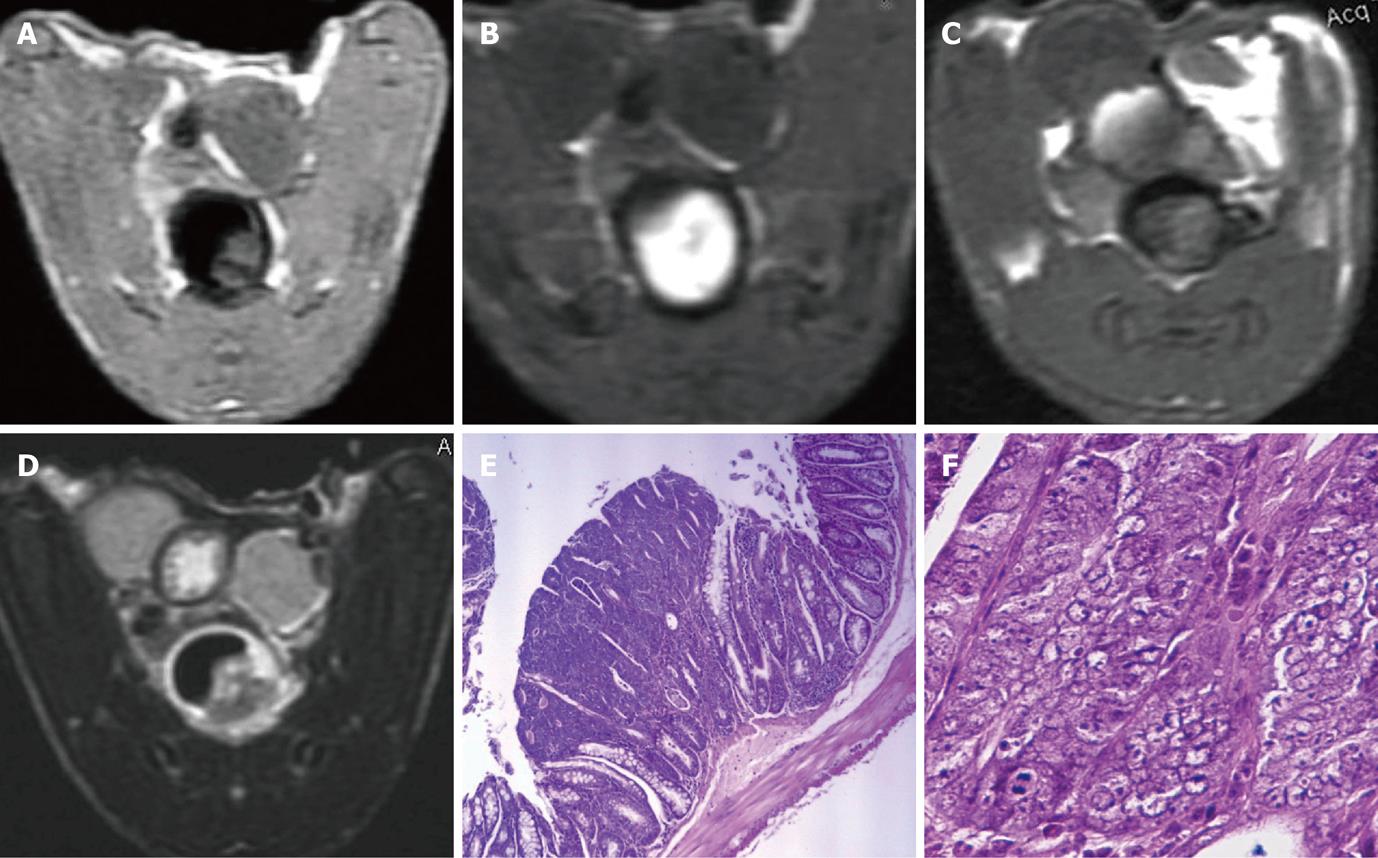
MR data of only 20 of the 30 DMH-treated mice were available due to DMH- and anesthesia-related mortality (n = 7) and operation failures (n = 3). In group 1, homogeneous enhancement and dense FITC deposition were observed in 1 mouse (8/10 slices) with no histological abnormity; the enhanced colorectal wall manifested as a bright rim sign on in-enema FLAIR images (Figure 2B). Heterogeneous enhancement was observed in 4 of the other 8 mice (26/48 slices). No visible mural enhancement was identified in the other 4 mice; mild dysplasia was identified in HE slices of the 8 mice with linear FITC deposition observed along the intestinal lumen in LSCFM images (Figure 3). In group 2, heterogeneous enhancement was shown in 2 of the 7 mice (6/16 slices); no other mural enhancement was observed. In group 3, no enhancement was found on FLAIR images after the FITC-SLN enema. Obvious dysplasia and intraepithelial neoplasia (low to high grade) were found in HE slices of mice receiving DMH for 16 wk (groups 2 and 3) with linear FITC deposition along the intestinal lumen observed in LSCFM images. Non-enhanced colorectal walls manifested as low signal rings around the “bright lumen” in in-enema FLAIR images, which was observed in 4 of the 7 mice in group 2 (Figure 4B).
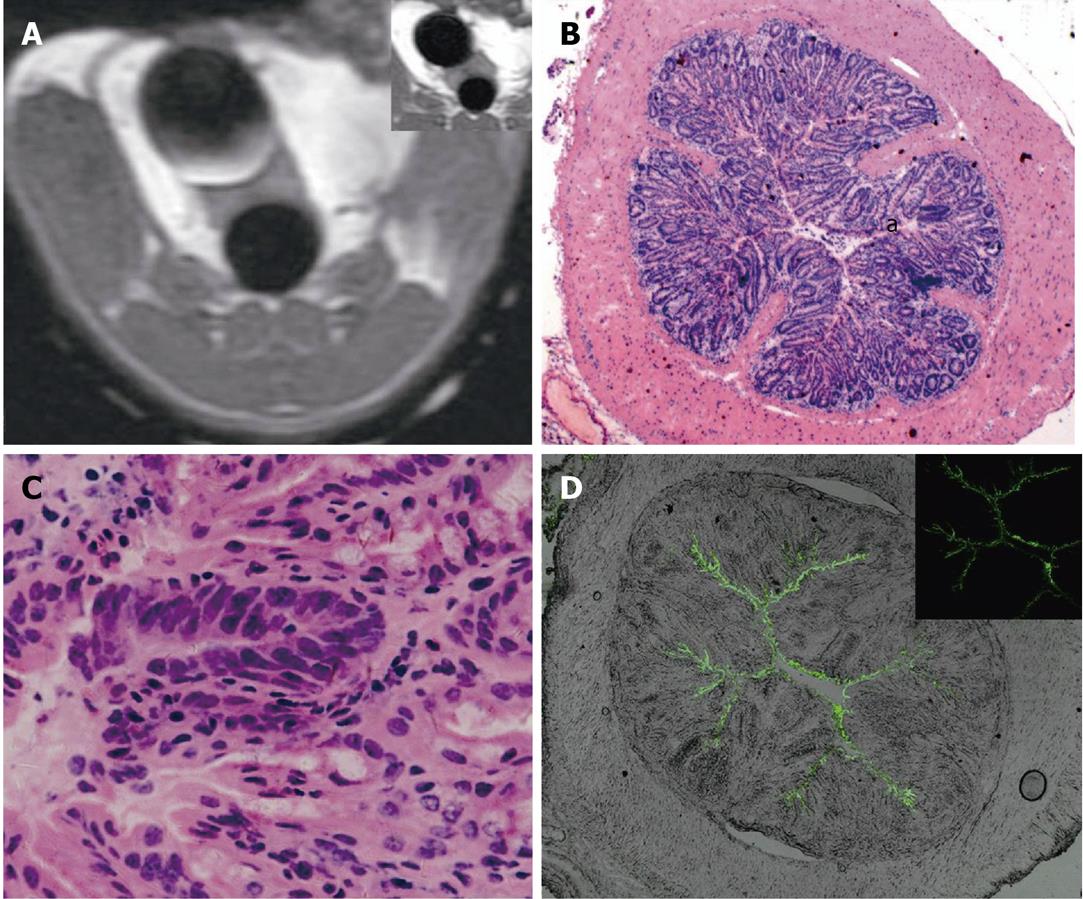
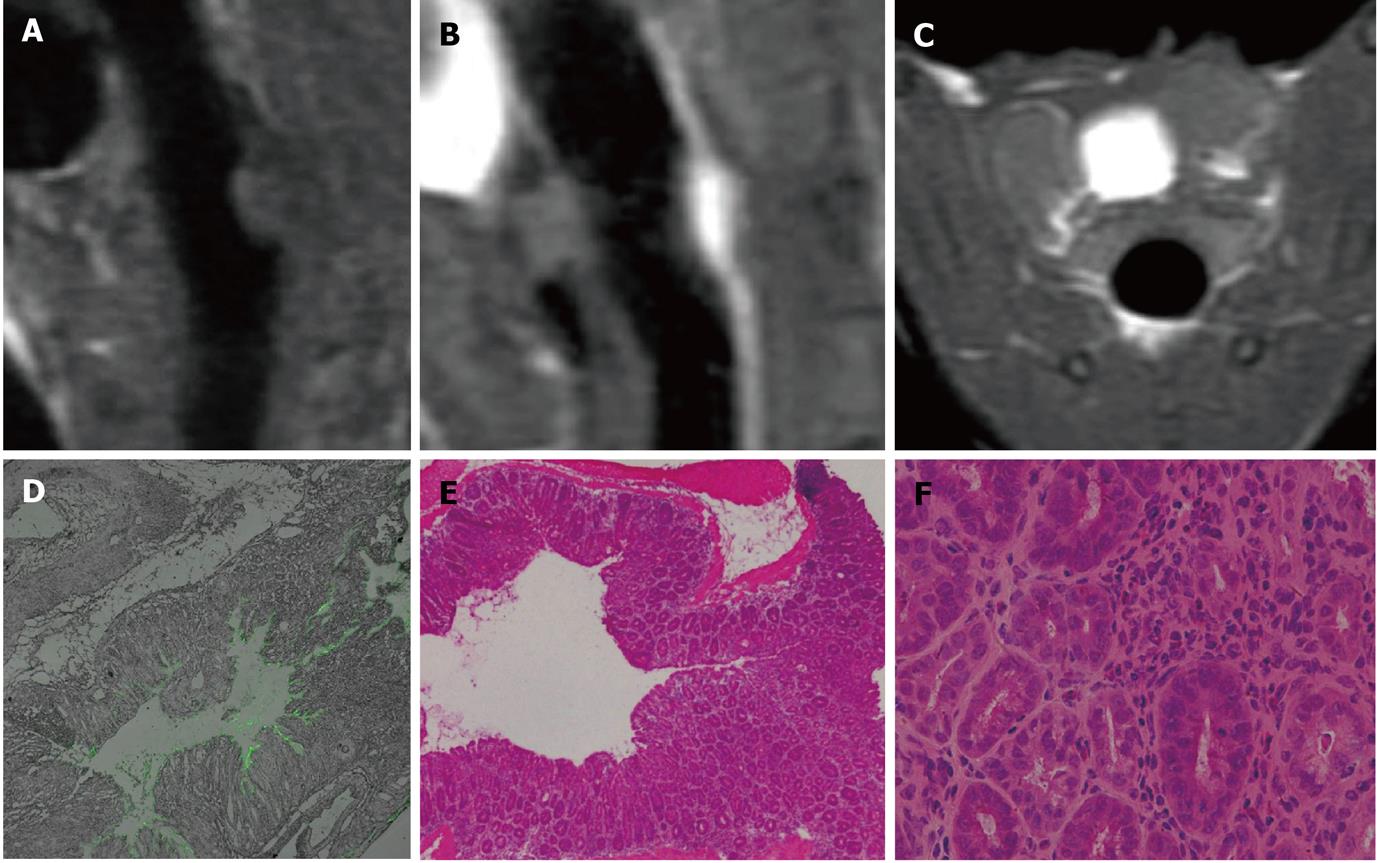
MR detected 9 intraluminal masses (short axis 2.06 ± 0.98 mm) and 1 prolapsed mass (well-differentiated squamous carcinoma) in groups 2 and 3. Eight of the 9 intraluminal masses were adenomas with different levels of malignancy; one of them was histologically proven as adenocarcinoma. The intraluminal masses manifested as filling deficits on Gd-FITC-SLN inflated bright-lumen FLAIR images. No visible enhancement was found in post-enema FLAIR images in one narrow-based adenoma with minimum FITC deposition along the edge and within the mass (Figure 5). Various degrees of enhancement were found in the other masses with interstitial FITC depositions in LSCFM images (Figure 6).
Colorectum stiffness was observed in 3 of 9 mice (groups 2 and 3) receiving DMH for 16 wk. Macroscopic evaluation of the animal’s distal colorectum revealed an extra adenoma measuring 2 × 3 mm in size in the sigmoid colon, which was missed in MR examination. No false-positive MR findings were observed by postmortem evaluation. Bladder imaging agent accumulation was accidently found in 11 of the 16 DMH-treated mice (groups 1 and 2) 3 - 28 (11.2 ± 9.6) min after the Gd-FITC-SLN enema was started (Figures 3-6).
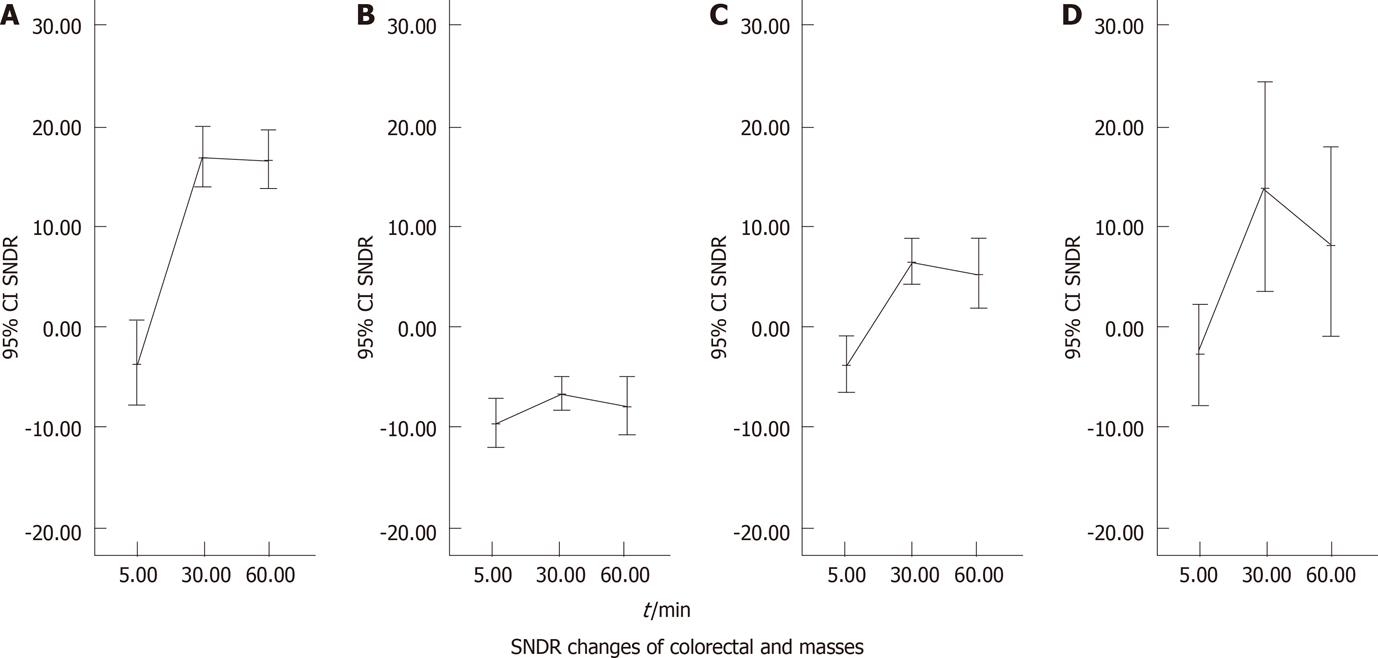
Pre- and post-enema SDNR values of colorectal wall in each group and intraluminal masses in DMH-treated mice were plotted against time (Figure 7) with brief annotation.
We used MR and LSCFM to study the colorectal uptake of SLNs. The bimodal imaging used elsewhere for tumor angiogenesis and lymphatic vessel imaging[17,18] can mutually confirm the information both macroscopically and microscopically. We used FLAIR for colorectal imaging. An early study reported that FLAIR sequences are more sensitive to low gadolinium concentrations than T1-weighted sequences[19]. Another study showed that post-contrast FLAIR imaging may improve the lesion depiction when a higher lesion SI exists on the T2-weighted images[20]. A set of methods for quantitative MR imaging analysis were evaluated, and a standardized method was proposed[16]. We followed the suggested procedure in terms of ROI definition and SDNR calculation.
We reviewed the studies on intestinal particulate substance uptake, colon-specific drug delivery and DMH-induced intestinal and renal impairment in order to explain the current results. It was proven, by lymph and plasma analysis, that more than 70% of the absorbed SLN was transported into systematic circulation via lymph, which is a major SLN transport pathway in the gastrointestinal tract[11]. Recent studies have further verified that the oral bioavailability of poorly water soluble contents (insulin, nitrendipine, tobramycin) increased significantly when encapsulated in the inner lipid matrix of SLNs[21,22].
The intestinal uptake of inert particles of the micrometer and nanometer range has been intensively studied in pharmaceutical research. The particles, usually used as oral drug delivery systems, include SLN (50-1000 nm), chitosan microspheres (2.1-12.5 μm), latex (2 μm), dendrimer and polymers, etc. The major conclusions of the studies[10,23-27] are summarized as follows. First, inert particulate uptake takes place along the entire length of the small and large intestines. Second, the process occurs not only via the M cells in the Peyer’s patches and the isolated follicles of the gut-associated lymphoid tissue, but also via the normal intestinal enterocytes. Third, factors affecting the uptake include particle size, surface charge and surface modification. Larger (micrometer range) and surface modified particles may be retained for longer periods in the Peyer’s patches, while smaller particles are transported to the thoracic duct. Fourth, an in vitro study using a Caco-2 cell model showed that 2 Gy X-irradiation increased particle (2 μm latex particles) uptake and translocation through the epithelium.
We hypothesize, based on the current results and earlier studies, that two different routes exist for the rectally administered SLN particles to enter the systematic circulation. In normal mice, the SLNs are mainly taken up by enterocytes and transferred to the lymphatic vessels and finally transported to the systematic circulation from the thoracic duct. In DMH-treated mice, however, the dominant route is from the carcinogen impaired colorectal mucosa, via the submucosal capillary network, to the mesenteric vein and then to the liver. As observed in this study, colorectal uptake and drainage of SLNs are an intracellular process; the transportation of SLNs in DMH-treated mice is a pathological process that occurs through an extracellular or interstitial route.
DMH and its metabolite azoxymethane (AOM) are specific colon carcinogens to model non-familial CRC in rodents[28]. Previous studies documented that DMH is also a renal carcinogen in mice[29-31]. The bladder gadolinium accumulation observed in this study may result from renal and intestinal epithelial impairment caused by DMH and its metabolites and the diuresis effect of manicol contained in SLN freeze-dried powder (20 mg /mL).
We believe that the bright rim sign in post-enema FLAIR images and corresponding cytoplastic FITC deposition in normal and group 1 mice is a manifestation of normal intestinal uptake function. Likewise, the halo sign observed in group 2 mice and linear extracellular FITC deposition is attributed to the impairment of intestinal epithelial barrier function. As functional changes always precede morphological lesions, further experiments are necessary to exemplify the early diagnostic potential by clarifying the mechanism at both cellular and molecular levels.
There were limitations in our study. First, layers of murine colorectal wall could not be differentiated on MR images due to the small animal size. We noticed that layers of the bowel and tumor invasion in the excised human colon cancer specimen were clearly depicted in one study[32]. Optimal delineation of layers of colorectal wall may likely be achieved if a porcine model was adopted. Second, sharp contrast in post-enema MR images (partial enhancement) existed but was rare, which may be explained by the diffuse impairment caused by DMH and its metabolite azoxymethane (AOM), delivered to the distal colorectum via the biliary system. Adoption of another colorectal tumor model, focally administered AOM, may improve the post-enema contrast between normal and cancerous tissues. Third, the long acquisition times of FLAIR pulse sequence may not be suitable for clinical MR colonography. More researches are, therefore, needed to optimize the technique in a clinical context.
High contrast between the bowel wall and lumen is essential for successful magnetic resonance (MR) colonography, which has been intensively studied in recent years. However, there has been no research aiming at MR visualizing the colorectal uptake of contrast medium.
In this study, colorectal uptake of solid lipid nanoparticles (SLNs) in normal and dimethylhydrazine (DMH)-treated mice was visualized by MR and laser-scanning confocal fluorescence microscopic imaging.
Significant differences in colorectal uptake and distribution of SLNs were revealed in normal and DMH-treated mice, which may provide new mechanisms of contrast for MR colonography.
Direct and in vivo imaging of colorectal uptake of nanoparticles could be translated into radiological and pharmaceutical applications. Further work is needed to explore the potential value of current findings for personalized therapy and radiographic follow-up.
SLNs are colloidal drug delivery systems with mean particle diameters ranging from 50 up to 1000 nm. SLNs combine the advantages of polymeric nanoparticles and fat emulsions for drug delivery administration, such as good tolerability, high oral bioavailability and large-scale production by high pressure homogenization. Magnetic resonance imaging is a cross-sectional imaging technique that does not utilize radiation and provides excellent tissue differentiation. MR colonography, based on the use of ultrafast MR sequences and relevant bowel contrast agents, is a less invasive colon imaging tool compared with optic colonoscopy.
The authors concluded that the uptake of SLNs into the colon wall was significant difference between normal and 1, 2-DMH, specific colon carcinogens, treated mice. This paper has very interesting results, but the objective is not clear.
| 1. | Geenen RW, Hussain SM, Cademartiri F, Poley JW, Siersema PD, Krestin GP. CT and MR colonography: scanning techniques, postprocessing, and emphasis on polyp detection. Radiographics. 2004;24:e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Ajaj W, Lauenstein TC, Pelster G, Holtmann G, Ruehm SG, Debatin JF, Goehde SC. MR colonography in patients with incomplete conventional colonoscopy. Radiology. 2005;234:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Kuehle CA, Langhorst J, Ladd SC, Zoepf T, Nuefer M, Grabellus F, Barkhausen J, Gerken G, Lauenstein TC. Magnetic resonance colonography without bowel cleansing: a prospective cross sectional study in a screening population. Gut. 2007;56:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Zhang S, Peng JW, Shi QY, Tang F, Zhong MG. Colorectal neoplasm: magnetic resonance colonography with fat enema-initial clinical experience. World J Gastroenterol. 2007;13:5371-5375. [PubMed] |
| 5. | Lauenstein TC, Goehde SC, Ruehm SG, Holtmann G, Debatin JF. MR colonography with barium-based fecal tagging: initial clinical experience. Radiology. 2002;223:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Martin DR, Yang M, Thomasson D, Acheson C. MR colonography: development of optimized method with ex vivo and in vivo systems. Radiology. 2002;225:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lauenstein TC, Schneemann H, Vogt FM, Herborn CU, Ruhm SG, Debatin JF. Optimization of oral contrast agents for MR imaging of the small bowel. Radiology. 2003;228:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Yeh P, Ellens H, Smith PL. Physiological considerations in the design of particulate dosage forms for oral vaccine delivery. Adv Drug Deliv Rev. 1998;34:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Reis CP, Ribeiro AJ, Houng S, Veiga F, Neufeld RJ. Nanoparticulate delivery system for insulin: design, characterization and in vitro/in vivo bioactivity. Eur J Pharm Sci. 2007;30:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Florence AT. The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm Res. 1997;14:259-266. [PubMed] |
| 11. | Yuan H, Chen J, Du YZ, Hu FQ, Zeng S, Zhao HL. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf B Biointerfaces. 2007;58:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Haupt S, Rubinstein A. The colon as a possible target for orally administered peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2002;19:499-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Reis CP, Veiga FJ, Ribeiro AJ, Neufeld RJ, Damgé C. Nanoparticulate biopolymers deliver insulin orally eliciting pharmacological response. J Pharm Sci. 2008;97:5290-5305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Uner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2:289-300. [PubMed] |
| 15. | Yuan H, Huang LF, Du YZ, Ying XY, You J, Hu FQ, Zeng S. Solid lipid nanoparticles prepared by solvent diffusion method in a nanoreactor system. Colloids Surf B Biointerfaces. 2008;61:132-137. [PubMed] |
| 16. | Pijl ME, Doornbos J, Wasser MN, van Houwelingen HC, Tollenaar RA, Bloem JL. Quantitative analysis of focal masses at MR imaging: a plea for standardization. Radiology. 2004;231:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49 Suppl 2:113S-128S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 382] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 18. | Mounzer R, Shkarin P, Papademetris X, Constable T, Ruddle NH, Fahmy TM. Dynamic imaging of lymphatic vessels and lymph nodes using a bimodal nanoparticulate contrast agent. Lymphat Res Biol. 2007;5:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Ercan N, Gultekin S, Celik H, Tali TE, Oner YA, Erbas G. Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery MR imaging of intracranial metastases. AJNR Am J Neuroradiol. 2004;25:761-765. [PubMed] |
| 20. | Kubota T, Yamada K, Kizu O, Hirota T, Ito H, Ishihara K, Nishimura T. Relationship between contrast enhancement on fluid-attenuated inversion recovery MR sequences and signal intensity on T2-weighted MR images: visual evaluation of brain tumors. J Magn Reson Imaging. 2005;21:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Kumar VV, Chandrasekar D, Ramakrishna S, Kishan V, Rao YM, Diwan PV. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007;335:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of nitrendipine solid lipid nanoparticles after intravenous and intraduodenal administration. J Drug Target. 2006;14:632-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Smyth SH, Feldhaus S, Schumacher U, Carr KE. Uptake of inert microparticles in normal and immune deficient mice. Int J Pharm. 2008;346:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Moyes SM, Killick EM, Morris JF, Kadhim MA, Hill MA, Carr KE. Changes produced by external radiation in parameters influencing intestinal permeability and microparticle uptake in vitro. Int J Radiat Biol. 2008;84:467-486. [PubMed] |
| 25. | Doyle-McCullough M, Smyth SH, Moyes SM, Carr KE. Factors influencing intestinal microparticle uptake in vivo. Int J Pharm. 2007;335:79-89. [PubMed] |
| 26. | Smyth SH, Doyle-McCullough M, Cox OT, Carr KE. Effect of reproductive status on uptake of latex microparticles in rat small intestine. Life Sci. 2005;77:3287-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50:107-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 429] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 28. | Bissahoyo A, Pearsall RS, Hanlon K, Amann V, Hicks D, Godfrey VL, Threadgill DW. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: effects of dose, route, and diet. Toxicol Sci. 2005;88:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Turusov VS, Lanko NS, Parfenov IuD, Chemeris GIu. [1,2-dimethylhydrazine induction of epithelial tumors of the kidneys in CBA strain mice]. Eksp Onkol. 1990;12:71-74. [PubMed] |
| 30. | Chemeris GIu, Poltoranina VS, Turusov VS. [Histogenesis of experimental renal tumors in mice]. Arkh Patol. 1992;54:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Turusov VS. Renal cell tumors induced in CBA male mice by 1,2-dimethylhydrazine. Toxicol Pathol. 1992;20:570-575. [PubMed] |
| 32. | Yamada I, Okabe S, Enomoto M, Sugihara K, Yoshino N, Tetsumura A, Kumagai J, Shibuya H. Colorectal carcinoma: in vitro evaluation with high-spatial-resolution 3D constructive interference in steady-state MR imaging. Radiology. 2008;246:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Peer reviewer: Reiji Sugita, MD, Departments of Radiology, Sendai City Medical Center, 5-22-1, Tsurugaya, Miyagino-ku, Sendai 983-0824, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Xiong L