Published online Aug 21, 2011. doi: 10.3748/wjg.v17.i31.3623
Revised: March 7, 2011
Accepted: March 14, 2011
Published online: August 21, 2011
AIM: To evaluate the antitumoral effect of combined inhibitors of angiogenesis and histone deacetylases in an experimental rat hepatoma model.
METHODS: MH7777A hepatoma cells were injected into the liver of male Buffalo rats. After 7 d treatment with the vascular endothelial growth factor receptor antagonist PTK787/ZK222584 (PTK/ZK), the histone deacetylase inhibitor MS-275, tamoxifen (TAM) and/or retinoic acid was initiated (n≥ 8 animals/group). Natural tumor development was shown in untreated control groups (control 1 with n = 12, control 2 with n = 8). The control groups were initiated at different time points to demonstrate the stability of the hepatoma model. For documentation of possible side effects, we documented any change in body weight, loss of fur and diarrhea. After 21 d treatment, the rats were euthanized. Main target parameters were tumor size and metastasis rate. Additionally, immunohistochemistry for the proliferating cell nuclear antigen (PCNA) and TdT-mediated dUTP-biotin nick end labeling (TUNEL) assay were performed.
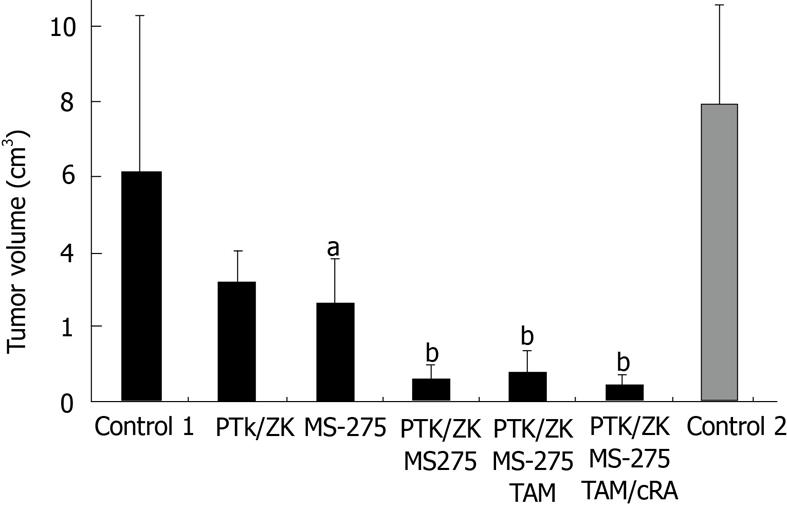
RESULTS: The control groups developed large tumor nodules with extrahepatic tumor burden in the lung and abdominal organs (control 1: 6.18 cm3± 4.14 cm3 and control 2: 8.0 cm3± 4.44 cm3 28 d after tumor cell injection). The tumor volume did not differ significantly in the control groups (P = 0.13). As single agents MS-275 and PTK/ZK reduced tumor volume by 58.6% ± 2.6% and 48.7% ± 3.2% vs control group 1, which was significant only for MS-275 (P = 0.025). The combination of MS-275 and PTK/ZK induced a nearly complete and highly significant tumor shrinkage by 90.3% ± 1% (P = 0.005). Addition of TAM showed no further efficacy, while quadruple therapy with retinoic acid increased antitumoral efficacy (tumor reduction by 93 ± 1%) and side effects. PCNA positive cells were not significantly reduced by the single agents, while dual therapy (MS-275 and PTK/ZK) and quadruple therapy reduced the PCNA-positive cell fraction significantly by 9.1 and 20.6% vs control 1 (P < 0.05). The number of TUNEL-positive cells, markers for ongoing apoptosis, was increased significantly by the single agents (control 1: 6.9%, PTK/ZK: 11.4%, MS-275: 12.2% with P < 0.05 vs control 1). The fraction of TUNEL-positive cells was upregulated highly significantly by dual therapy (18.4%) and quadruple therapy (24.8%, P < 0.01 vs control 1). For the proliferating (PCNA positive) and apoptotic cell fraction, quadruple therapy was significantly superior to dual therapy (P = 0.01).
CONCLUSION: Combined PTK/ZK and MS-275 were highly effective in this hepatoma model. Quadruple therapy enhanced the effects microscopically, but not macroscopically. These results should be investigated further.
- Citation: Ganslmayer M, Zimmermann A, Zopf S, Herold C. Combined inhibitors of angiogenesis and histone deacetylase: Efficacy in rat hepatoma. World J Gastroenterol 2011; 17(31): 3623-3629
- URL: https://www.wjgnet.com/1007-9327/full/v17/i31/3623.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i31.3623
Hepatocellular carcinoma (HCC) is one of the most common tumor entities in Asia and Africa, but not in western countries[1,2]. However, the increasing rate of hepatitis C virus infection and alcohol abuse has led to a rising incidence of HCC in industrial countries[1,2].
Life-prolonging HCC therapy is based on surgical tumor elimination (liver transplant, resection) or interventional local ablation[3,4]. For large tumor volume or metastasis, therapeutic options are limited, because HCC is resistant to systemic chemotherapy[3,4]. A wide range of experimental compounds, such as vitamin D or tamoxifen (TAM) were not effective in placebo-controlled trials[1,3,4]. Therefore, not single, but combination treatment should be evaluated. We have shown that combined application of TAM and cis-retinoic acid (CRA) induces moderate antitumoral effects in a rat model, while single agents are ineffective[5,6]. Current investigations have put combination molecular targeted therapy into focus[7-9]. Sorafenib is a molecular targeted agent with antiproliferative as well as antiangiogenic activity. It is the first effective compound in HCC patients, which proves the concept of combination treatment. However, the antitumoral and life-prolonging effects of sorafenib are very limited[3,7-9]. Nevertheless, the strategy of combined molecular targeted agents has to be further investigated.
In most HCCs, a high grade of vascularity has been demonstrated. Folkman et al[10-12] have demonstrated that any tumor larger than a few millimeters in diameter induces angiogenesis for self-supplementation. Furthermore, connection with the host’s vessel system and degradation of the extracellular matrix leads to metastasis[11]. Therefore, inhibitors of angiogenesis have been shown to reduce hepatoma volume in vivo and in vitro[9-12]. In recent years, highly effective synthetic angiogenesis inhibitors, such as PTK787/ZK222584 (PTK/ZK), have been developed for cancer therapy. These are currently being evaluated in clinical trials or approved for therapy of advanced colorectal cancer[12,13].
Histone deacetylase (HDAC) inhibitors suppress the post-translational deacetylation of histone proteins. In consequence, these histone proteins are hyperacetylated and the DNA structure is loosened, which mediates enhanced binding of transcription factors to certain gene loci and higher gene expression[14]. HDAC inhibitors have been shown to induce growth inhibitory genes and pro-apoptotic factors in vivo and in vitro[7,14,15]. MS-275 is a benzamide with activity against HDAC class 1 and 2. Ongoing phase I-III trials have displayed no adverse effects, therefore, further clinical development is ongoing[16,17].
In this experimental setting, we evaluated a combination of the angiogenesis inhibitor PTK/ZK and the HDAC inhibitor MS-275[12,16] in a syngeneic rat model of hepatoma. PTK/ZK is an aminophthalocine and a potent inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases, which impairs VEGF-induced responses and tumor growth after oral administration (Vatalanib®)[12,16,18]. VEGF receptors mediate an increase in vascular permeability, angiogenesis and lymphogenesis. Mainly, the expression of VEGF receptor 1 is enhanced in tumor tissue[16,18]. PTK/ZK inhibits VEGF receptors 1-4 with highest potency towards receptors 1 and 2. Additionally, we added TAM and CRA, which have been shown to act via intracellular receptors and to be effective combination partners in this HCC model[5].
For in vivo experiments MS-275 was suspended in methanol, while 9-cis retinoic acid (cRA), TAM and PTK787/ZK222584 (Vatalanib®, PTK/ZK) were dissolved in DMSO. Any of these stock solutions were diluted at least 20-fold with Aqua injectabile (Baxter, Deerfield, IL, United States) to a final maximum concentration of 5% DMSO or 0.5% methanol before injection. MS-275 and PTK/ZK were kindly donated by Schering AG (Berlin, Germany).
Male Buffalo rats (200-350 g) from Charles River Laboratories (Schweinfurt, Germany) were kept as pairs in polycarbonate cages (Eurostandard III H; Techniplast, Berlin, Germany). Room temperature was kept at 27 °C and room humidity maintained at 30%. The rats were fed a standardized gluten-poor diet (Altromin, Frankfurt, Germany) and water. Animal maintenance and experimental procedures were approved by the government of middle Franconia and carried out according to “The 1996 Guide for the Care and Use of Laboratory Animals” as published in ILAR[19].
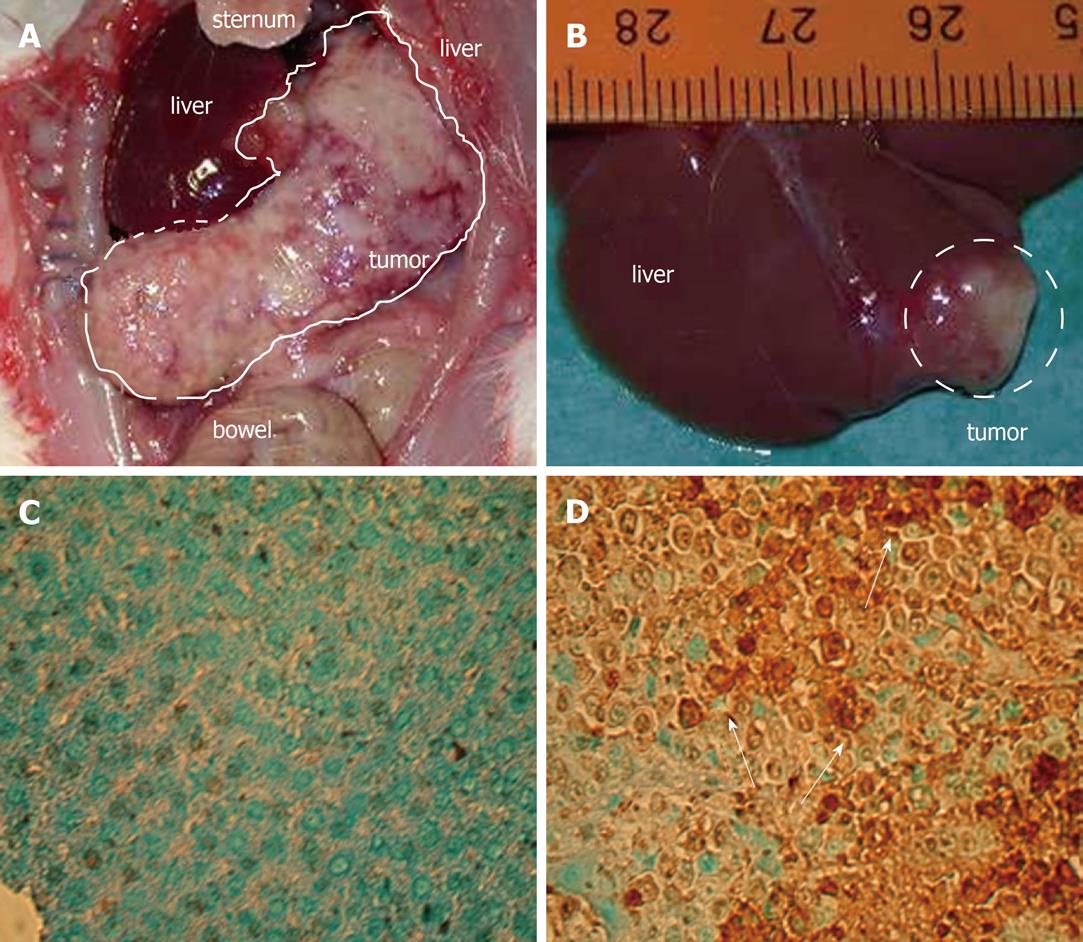
For tumor induction, MH7777A cells (DSMZ, Braunschweig, Germany) were grown in Dulbecco’s Modified Eagle’s Medium (Biochrom, Berlin, Germany) that contained 10% fetal calf serum (Gibco BRL, Karlsruhe, Germany), penicillin (100 U/L), streptomycin (10 mg/L), insulin and dexamethasone at 37 °C under 5% CO2 (5-10 passages). The cells were trypsinized, suspended in PBS (Biochrom) at a concentration of 106 cells/100 μL. Buffalo rats were anesthetized using ethyl ether. After a median laparotomy (2 cm), the liver was embedded into wet sterile compresses. One hundred microliters of the cell suspension was injected into the subcapsular space of the left liver lobe and leakage of tumor cells was prevented by compression and a hemostatic (Tabotamp; Ethicon, Johnson and Johnson, Norderstedt, Germany). The animals received metamizol for analgesia (7 d). They were controlled for diarrhea, loss of hair, food intake and unusual behavior daily, and body weight was measured weekly. On postoperative day 7 (tumor size 5-7 mm diameter), treatment with single or combined drugs was started. The drugs were administered at the recommended dose of 50 mg/kg per day i.p. for PTK/ZK, 3 mg/kg per day i.p. for MS-275, 10 mg/kg per day i.p. for TAM and 6 mg/kg per day i.p. for CRA. After 21 d treatment (day 28 after tumor implantation), the rats were euthanized with ether anesthesia. At least eight animals were evaluated per group.
The liver was removed and the tumor volume calculated using the formula (largest diameter a × smallest diameter b2)/2 as recommended in the literature[6]. The following organs were inspected for tumor nodules: lungs, spleen, kidneys, peritoneum and diaphragm. The primary tumor and both lungs were fixed in 5% buffered formalin.
TdT-mediated dUTP-biotin nick end labeling (TUNEL)-positive cells were analyzed using the in situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, formalin-fixed tissues were permeabilized with proteinase K (30 min, 37 °C) and peroxidase blocked in methanol containing 0.3% H2O2. Fluorescent nucleotides mixed with terminal deoxynucleotide transferase were added for 60 min at 37 °C, followed by incubation with converter-peroxidase (POD) conjugated anti-fluorescein antibody (provided in the kit) for 30 min at 37 °C. Slides were developed using diaminobenzidine (DAB) substrate for 10 min and counterstained using methylene green (7.5%, 7 min at room temperature).
Proliferating cell nuclear antigen (PCNA)-positive cells in formalin-fixed tissue were detected after blocking of endogenous biotin with chicken egg and 1.5% fat milk for 15 min at room temperature. Mouse PCNA antibody (Novo Laboratories, Newcastle, United Kingdom) was diluted 1:50 in Tris buffer and added for 2 h, followed by 30 min incubation with the biotinylated second antibody. Color was developed with streptavidin-alkaline phosphatase complex (DAKO, Mannheim, Germany) and FAST Red (Sigma, Frankfurt, Germany).
The stained sections were examined using a light microscope (Axiophot, Nikon coolpix 99; Zeiss, Jena, Germany) and the CellExplorer 2001 software (BioSciTec, Frankfurt/Main, Germany). For quantification of TUNEL- and PCNA-positive cells, 10 high power fields per slide were investigated at 400 × magnification. Four of eight animals were analyzed per experimental group. All cell nuclei were related to the specifically stained cells to obtain the percentage of positive cells per slide.
For qualitative validation of the anti-angiogenic activity cryofixed sections (6 μm, lysine-coated slides) were blocked (buffer containing 2% BSA, 0.2% low fat milk, 2% mouse serum and PBS) and incubated with a rabbit anti-von Willebrand factor (vWF) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States; 1:200 dilution) for 1 h at 37 °C. After several washing steps and addition of a biotinylated second antibody (30 min, room temperature), color was developed with streptavidin–peroxidase complex (DAKO) and DAB. Counterstaining was done using standard haemalaun.
Statistical analysis was performed using SPSS for Windows version 16.0. Significance was calculated using the t test or Wilcoxon test for paired samples (if not otherwise stated vs control 1). P < 0.05 was regarded as significant, and P < 0.01 as highly significant.
In untreated animals (control 1, n = 12) the tumor volume was 6.18 cm3± 4.14 cm3 after 28 d. In a second untreated control group (control 2, n = 8), which was started at an independent time point, tumor volume was 8.0 cm3± 4.44 cm3. This was not significantly different to control 1 (P = 0.13) and may confirm the reproducibility of the animal model.
Twenty-eight days after tumor implantation 80%-90% of the animals suffered from diffuse lung metastases and tumor spread to kidneys, spleen and peritoneum. Eighty percent of the animals developed ascites. Bleeding from the eyes and the nose showed impaired coagulation. The body weight was increased by 17% ± 15% (due to ascites and tumor burden).
As stated before, treatment with TAM or CRA as single agents showed no significant antitumoral efficacy, while a combination of both showed moderate tumor reduction (14%, 8.5% and 60% for TAM, CRA and TAM/CRA)[7].
Even the single agents PTK/ZK and MS-275 induced some antitumoral effects and reduced tumor volume by 50 and 60% (Figure 1). However, PTK/ZK failed to show significant antitumoral efficacy (P = 0.212), while MS-275 showed significant tumor growth reduction even as a single agent (P = 0.025). Dual therapy with PTK/ZK and MS-275 reduced tumor growth by > 90% in a highly significant manner (P = 0.005, Figures 1 and 2). PTK/ZK + MS-275 + TAM showed no additional effect on tumor volume, while the quadruple therapy enhanced the efficacy slightly, but not significantly (P = 0.007 and 0.002 for triple and quadruple therapy vs control 1; P = 0.49 and P = 0.039 vs dual therapy, Figure 1).
Monotherapy did not change the extent of extrahepatic tumor burden, while combination therapy reduced rate of metastases significantly (Table 1). Again, quadruple therapy failed to enhance the effects of combined PTK/ZK + MS-275 (data not shown).
| Control group (n = 12) | Monotherapy (n = 17) | Combination therapy (n = 24) | P value2 | |
| Tumor burden - pulmonary | 83.3 | 82.4 | 70.8 | 0.586 |
| Tumor burden - abdominal | 91.7 | 100.0 | 66.7 | 0.013 |
| Ascites | 83.3 | 29.4 | 0.0 | < 0.001 |
| Behavior1 | 41.7 | 0.0 | 25.0 | 0.019 |
| Loss of fur | 16.7 | 41.2 | 66.7 | 0.015 |
| Loss of weight (> 10% body weight) | 0.0 | 47.1 | 79.2 | < 0.001 |
| Diarrhea | 16.7 | 29.4 | 62.5 | 0.015 |
Compared to placebo treatment, monotherapy increased the rate of diarrhea and loss of fur. Combination therapy intensified the number of these side effects significantly (Table 1). Subgroup analysis showed fewer side effects for dual therapy compared to triple and quadruple therapy. However, this particular result was not significant due to the low number of animals/group (data not shown).
Any effective treatment (monotherapy, dual therapy and quadruple therapy) induced loss of weight, which can be explained by the increased rate of diarrhea and the reduced amount of ascites in these treatment groups (Table 1). No animal had to be euthanized due to the number or severity of side effects.
After treatment with PTK/ZK alone and in combination, vessel density decreased qualitatively, which was exemplified by staining with vWF antibody in a subgroup of animals (data not shown). Quantification of microvessel density was not performed, because the antiangiogenic efficacy of PTK/ZK has been well described[12,19].
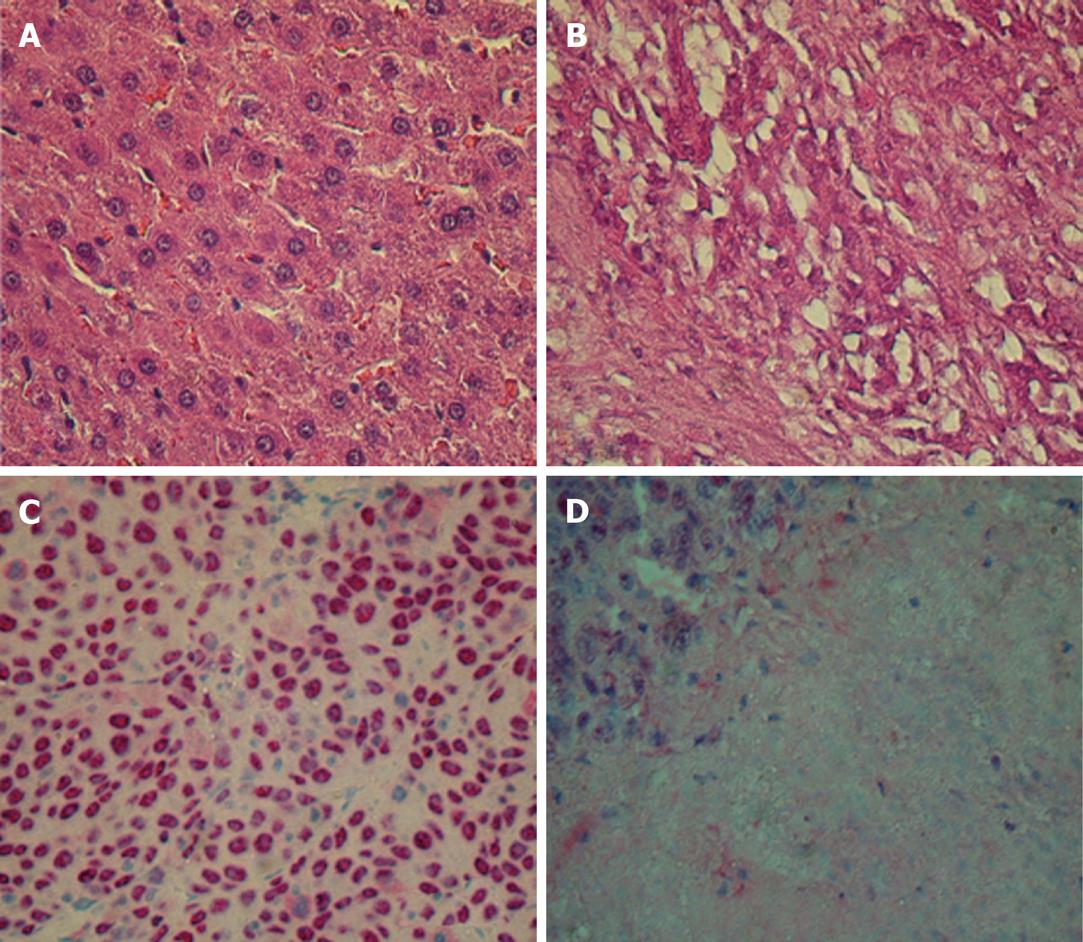
In hematoxylin and eosin (HE) and immunohistochemically stained tissue, any effective treatment went along with an increase in areas of necrosis (Figure 3). Depending on the extent of the necrotic areas, we detected 200-500 cells/field. The number of TUNEL-positive cells as a marker for ongoing apoptosis increased significantly after monotherapy (P = 0.04 and 0.02 for PTK/ZK and MS-275 vs control 1). As expected, the number of apoptotic cells increased even more markedly after dual therapy (highly significant vs control 1 with P = 0.002). The results of quadruple therapy were significantly higher even if compared to dual therapy (P = 0.01 for quadruple therapy vs dual therapy; Table 2). The signal for proliferating cells (PCNA positive) remained stable for monotherapy, but decreased significantly for dual therapy (P = 0.04 vs controls). Again, quadruple therapy showed a significant difference compared to the combination of PTK/ZK + MS-275 (P = 0.01 for quadruple vs dual therapy) (Table 2).
Extensive screening procedures and better imaging techniques have increased the mean survival time of HCC patients[2-4]. The curative or life-prolonging therapeutic options are based on tumor ablation via liver transplantation, resection or local instillation of heat or ethanol[3,4]. To date, sorafenib is the only systemic treatment option. Sorafenib acts via antiproliferative and antiangiogenic effects and is the proof of concept for systemic treatment in HCC. However, the achieved life-prolonging effect of a few months is a very limited benefit, and a wide range of side effects is induced[7-9]. The majority of patients have advanced-stage tumors and are beyond effective therapy when diagnosed[3,4].
Basic science studies have shown that HCC develops distinct molecular changes and patterns. It has been hypothesized that a couple of mutations leads to a change from cirrhotic nodules to invasive carcinoma. Mutations are observed for the tumor suppressor gene p53 or for members of signal transduction pathways such as insulin receptor substrate-1 or β-catenin[2,20-22]. Different subtypes of HCC have been classified. Each subtype displays distinguishable genetic alterations and characteristics in clinical behavior[20-22].
This may explain why systemic treatment with a single agent fails to be effective for HCC, and why some compounds display their antitumoral efficacy only in specific subgroups of HCCs. Therefore, combination therapy should represent a possible treatment option as shown in other malignancies, such as colorectal cancer. We have shown that HDAC inhibitors combined with retinoids or conventional chemotherapeutic agents induce apoptosis and decrease growth of hepatoma cells in an additive manner[5,23]. Furthermore, we have confirmed that retinoids or TAM as monotherapy have no effect in vivo, while the combined agents are moderately effective[7].
In the current setting, we evaluated combination therapy in a syngeneic rat hepatoma model. HCC is known to be highly vascularized and to produce a wide range of proangiogenic factors[4,10]. In an experiment by Yao et al[24], 70% of resected HCC nodules showed increased expression of VEGF, which correlates with metastasis rate and poor prognosis. Therefore, we chose PTK/ZK, which selectively inhibits the tyrosine kinase domains of VEGF receptors, platelet-derived growth factor receptors and c-KIT[12,18,24]. PTK/ZK is an accepted antiangiogenic partner in the treatment of colorectal cancer. In the preceding clinical evaluation only minor adverse effects occurred, such as headache, vertigo and arterial hypertension[12].
Here, we observed a remarkable but nonsignificant effect with reduction of tumor burden. As expected, no animal was cured by monotherapy with PTK/ZK. Inhibition of angiogenesis does not reduce the tumor mass completely. Small aggregations of malignant cells can exist without a vessel system[11], therefore, inhibition of angiogenesis can never represent a monotherapeutic option.
As a combination partner we chose MS-275, an HDAC inhibitor. HDAC inhibitors are known to change gene expression via hyperacetylation of histones, which are transcription-regulatory intranuclear proteins. Subsequently, upregulation of genes induces growth arrest and cell differentiation and maturation (e.g. p21, transforming growth factor β and gelsolin)[5,14-17]. Therefore, HDAC inhibitors may be of value in antitumoral therapy, as shown in vitro and in vivo[14]. MS-275 has shown acceptable results in phase I trials and has proceeded to phase II evaluation[16,25].
In the current experimental setting, the single agent MS-275 showed significant antitumoral effects. Combination with PTK/ZK induced an excellent reduction of tumor volume in this aggressive tumor model, which was even highly significant when compared to the effects of the single agents. Histological evaluation showed necrotic areas as a sign of tumor destruction. An unproved explanation could be an increase of toxic radicals and reduced oxygen supplementation. PCNA staining and TUNEL assay confirmed the superiority of dual therapy. This supports the hypothesis that combination therapy exceeds the efficacy of monotherapy significantly and should be further evaluated.
Since HDAC inhibitors are known to interfere with intracellular retinoid and estrogen receptors and to enhance their antiproliferative effects at least in vitro[4,5,21], we decided to evaluate triple and quadruple therapy. The combination of PTK/ZK + MS-275 + TAM did not increase the macroscopic antitumoral effect compared to dual therapy.
Quadruple therapy (PTK/ZK + MS-275 + TAM + CRA) induced a slight, but nonsignificant benefit in tumor volume, while the results for PCNA staining and TUNEL assay were enhanced significantly. Additionally, HE staining revealed large necrotic areas in these tumor samples (after quadruple therapy). Similar results have been reported for VEGF and epidermal growth factor inhibition in other tumor entities (e.g. colorectal cancer), which did not reduce the absolute tumor volume, but increased the areas of necrosis within the tumor[11,12]. Unfortunately a 3D analysis of the necrotic tumor regions in untreated controls vs animals with single or combined treatment was not done in this study. We can only postulate a similar mechanism and recommend dynamic imaging (magnetic resonance imaging or computed tomography) for the estimation of necrotic vs vital tumor regions in future studies. The same goes for the effects on angiogenesis: due to the proven antiangiogenic effect of PTK/ZK, we did not quantify the microvessel density. However, effects of certain histone deacetylases on the extracellular matrix have recently been shown[26]. Therefore, the changes in microvessel density after combination therapy compared to those with PTK/ZK monotherapy would be particularly interesting, and could explain the enhanced effects of combination therapy.
Analysis of the side effects showed diarrhea and loss of fur in animals treated with single agents, which was intensified by combined therapy. Subgroup analysis did not reach significance, but showed fewer side effects for dual therapy compared to triple and quadruple therapy. The observed loss of weight may be explained by diarrhea and the reduced amount of ascites after tumor treatment. Altogether, no single or combined treatment induced unacceptable side effects.
The relatively small number of animals in this study did not allow evaluation of the increased side effect profile vs the additional benefit of triple and quadruple therapy. Investigations with a higher number of animals and a longer treatment period are necessary to assess the benefit of this quadruple therapy vs dual therapy.
In summary, we showed that combination therapy is superior to monotherapy. At least in this rat model for HCC, PTK/ZK and MS-275 were highly effective, which justifies further investigation. The antitumoral effects were seen by macroscopic evaluation of tumor volume and evaluation of proliferation and apoptotic cells, which was especially marked in relation to decreasing tumor mass. The effects of triple and quadruple therapy need to be analyzed in further experiments. In the next step, the efficacy of dual therapy should be evaluated in different genetic, well-defined hepatoma models, which could possibly provide insight into the triggered pathways. If dual therapy (PTK/ZK and MS-275) is successful in this additional experimental setting, clinical development seems feasible.
The incidence of hepatocellular carcinoma (HCC) is increasing. Curative and life-prolonging therapeutic options for early tumor stages are resection, transplantation and interventional treatment. Sorafenib is the first systemic treatment option. However, the life-prolonging effect of sorafenib is limited to a few months. Effective systemic treatment for far-advanced hepatoma is still lacking.
Tumor cells show signs of dedifferentiation, reduced apoptosis, and an increase in proliferation rate. They induce angiogenesis and changes in the extracellular fibers. Biomodulators are directed against these tumor-cell-specific patterns. Histone deacetylase (HDAC) inhibitors change the expression of proliferation and apoptosis-inducing factors, and lead to normalization of protein expression in tumor cells. Vascular endothelial growth factor (VEGF) receptor antagonists reduce tumor-cell-induced neoangiogenesis and destruction of the extracellular matrix. The antitumoral efficacy of these biomodulators, such as HDAC inhibitors and VEGF receptor blockers, can be evaluated in vitro and in vivo. The authors showed that combination therapy was far superior to monotherapy in vitro (proliferation rate, induction of apoptosis). However, to date, there are not sufficient data to prove this principle in vivo. We used a syngeneic rat model. Morris hepatoma cells were implanted into the liver. The endpoint was macroscopic tumor growth and microscopic changes in proliferation, apoptosis and chemotaxis.
The authors evaluated monotherapy and combination therapy with four different agents: the HDAC inhibitor MS-275, the VEGF receptor blocker PTK787/ZK222584 (PTK/ZK), tamoxifen and retinoic acid. We reported significant antitumoral efficacy. Combined treatment was superior to the single agents. The side effect profile was acceptable even after combination therapy.
Combination therapy should be compared to the gold standard sorafenib in an in vivo model. The agents have been well described, and the next step could be a phase I trial.
In this study, the authors examined the effects of combination therapy using PTK/ZK and MS-275 in a rat HCC model, and showed that combined therapy was highly effective. This study is significant because development of systemic chemotherapy for advanced HCC is an important subject.
| 1. | El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37 Suppl 2:S88-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 3. | Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Ganslmayer M, Ocker M, Zopf S, Leitner S, Hahn EG, Schuppan D, Herold C. A quadruple therapy synergistically blocks proliferation and promotes apoptosis of hepatoma cells. Oncol Rep. 2004;11:943-950. [PubMed] |
| 5. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 833] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 6. | Ganslmayer M, Ocker M, Kraemer G, Zopf S, Hahn EG, Schuppan D, Herold C. The combination of tamoxifen and 9cis retinoic acid exerts overadditive anti-tumoral efficacy in rat hepatocellular carcinoma. J Hepatol. 2004;40:952-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Richly H, Schultheis B, Adamietz IA, Kupsch P, Grubert M, Hilger RA, Ludwig M, Brendel E, Christensen O, Strumberg D. Combination of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma: results from a phase I extension trial. Eur J Cancer. 2009;45:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 10. | Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15-18. |
| 11. | Thomas AL, Trarbach T, Bartel C, Laurent D, Henry A, Poethig M, Wang J, Masson E, Steward W, Vanhoefer U. A phase IB, open-label dose-escalating study of the oral angiogenesis inhibitor PTK787/ZK 222584 (PTK/ZK), in combination with FOLFOX4 chemotherapy in patients with advanced colorectal cancer. Ann Oncol. 2007;18:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ellis LM, Rosen L, Gordon MS. Overview of anti-VEGF therapy and angiogenesis. Part 1: Angiogenesis inhibition in solid tumor malignancies. Clin Adv Hematol Oncol. 2006;4:suppl 1-10; quz 11-2. [PubMed] |
| 13. | Fang JY. Histone deacetylase inhibitors, anticancerous mechanism and therapy for gastrointestinal cancers. J Gastroenterol Hepatol. 2005;20:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Herold C, Ganslmayer M, Ocker M, Hermann M, Geerts A, Hahn EG, Schuppan D. The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J Hepatol. 2002;36:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, Chen M, Steinberg SM, Muir CA, Yancey MA. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur J Cancer. 2010;46:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Qu W, Kang YD, Zhou MS, Fu LL, Hua ZH, Wang LM. Experimental study on inhibitory effects of histone deacetylase inhibitor MS-275 and TSA on bladder cancer cells. Urol Oncol. 2009;28:648-654. [PubMed] |
| 17. | Liu Y, Poon RT, Li Q, Kok TW, Lau C, Fan ST. Both antiangiogenesis- and angiogenesis-independent effects are responsible for hepatocellular carcinoma growth arrest by tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2005;65:3691-3699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997;38:41-48. [PubMed] |
| 19. | Wong CM, Ng IO. Molecular pathogenesis of hepatocellular carcinoma. Liver Int. 2008;28:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Matsuda Y. Molecular mechanism underlying the functional loss of cyclindependent kinase inhibitors p16 and p27 in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ocker M, Alajati A, Ganslmayer M, Zopf S, Lüders M, Neureiter D, Hahn EG, Schuppan D, Herold C. The histone-deacetylase inhibitor SAHA potentiates proapoptotic effects of 5-fluorouracil and irinotecan in hepatoma cells. J Cancer Res Clin Oncol. 2005;131:385-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, Wu W, Qiu LW, Meng XY. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220-226. [PubMed] |
| 23. | Hauschild A, Trefzer U, Garbe C, Kaehler KC, Ugurel S, Kiecker F, Eigentler T, Krissel H, Schott A, Schadendorf D. Multicenter phase II trial of the histone deacetylase inhibitor pyridylmethyl-N-{4-[(2-aminophenyl)-carbamoyl]-benzyl}-carbamate in pretreated metastatic melanoma. Melanoma Res. 2008;18:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Wang XF, Qian DZ, Ren M, Kato Y, Wei Y, Zhang L, Fansler Z, Clark D, Nakanishi O, Pili R. Epigenetic modulation of retinoic acid receptor beta2 by the histone deacetylase inhibitor MS-275 in human renal cell carcinoma. Clin Cancer Res. 2005;11:3535-3542. [PubMed] |
| 25. | Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med. 1999;340:1046-1047. [PubMed] |
| 26. | Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. [PubMed] |
Peer reviewers: Yoshihisa Takahashi, MD, Department of Pathology, Teikyo University School of Medicine, 2-11-1 Kaga, Itabashi-ku, Tokyo 173-8605, Japan
S- Editor Sun H L- Editor Kerr C E- Editor Xiong L