Published online Aug 21, 2010. doi: 10.3748/wjg.v16.i31.3957
Revised: April 27, 2010
Accepted: May 4, 2010
Published online: August 21, 2010
AIM: To analyze the accuracy of computed tomography (CT) angiography in the diagnosis of acute gastrointestinal (GI) bleeding.
METHODS: The MEDLINE, EMBASE, Cancerlit, Cochrane Library database, Sciencedirect, Springerlink and Scopus, from January 1995 to December 2009, were searched for studies evaluating the accuracy of CT angiography in diagnosing acute GI bleeding. Studies were included if they compared CT angiography to a reference standard of upper GI endoscopy, colonoscopy, angiography or surgery in the diagnosis of acute GI bleeding. Meta-analysis methods were used to pool sensitivity and specificity and to construct summary receiver-operating characteristic.
RESULTS: A total of 9 studies with 198 patients were included in this meta-analysis. Data were used to form 2 × 2 tables. CT angiography showed pooled sensitivity of 89% (95% CI: 82%-94%) and specificity of 85% (95% CI: 74%-92%), without showing significant heterogeneity (χ2 = 12.5, P = 0.13) and (χ2 = 22.95, P = 0.003), respectively. Summary receiver operating characteristic analysis showed an area under the curve of 0.9297.
CONCLUSION: CT angiography is an accurate, cost-effective tool in the diagnosis of acute GI bleeding and can show the precise location of bleeding, thereby directing further management.
- Citation: Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: A meta-analysis. World J Gastroenterol 2010; 16(31): 3957-3963
- URL: https://www.wjgnet.com/1007-9327/full/v16/i31/3957.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i31.3957
Acute gastrointestinal (GI) bleeding represents a common medical emergency with an annual incidence of 40-150 episodes per 100 000 persons for upper GI hemorrhage and 20-27 episodes per 100 000 persons for lower GI hemorrhage[1]. GI bleeding is usually classified as upper or lower based on whether the bleeding source is proximal or distal to the ligament of Treitz[2].
Nearly 75% of bleeding will cease spontaneously, however, bleeding can recur in 25% of cases, causing significant morbidity and mortality[3,4]. Mortality rates are generally 3%-5%, but can reach up to 23% with massive bleeding or bleeding that recurs after hospitalization[5].
There are multiple modalities that are currently being used in the evaluation and treatment of acute GI hemorrhage, each with its own strengths and weaknesses[6,7].
Esophagogastroduodenoscopy (EGD) and colonoscopy are currently considered the first-line diagnostic procedures of choice for both upper and lower GI bleeding[8,9]. Endoscopic evaluation allows relatively safe, direct localization and characterization of bleeding lesions within the majority of the upper GI tract as well as in the colon and distal ileum. The distal duodenum and the majority of the small bowel cannot be adequately assessed with conventional endoscopy. The reported sensitivity and specificity of EGD for upper GI bleeding are 92%-98% and 30%-100%, respectively[4].
Noninvasive imaging with technetium-99m (Tc-99m)-labeled red blood cell (RBC) or Tc-99m sulfur colloid scintigraphy can be used to detect and localize GI bleeding. Tc-99m RBC scintigraphy is 93% sensitive and 95% specific for detecting a bleeding site with active arterial or venous bleeding rates as low as 0.2 mL/min[10] anywhere within the GI tract. An advantage of red cell scintigraphy is the ability to carry out delayed scans up to 24 h after radioisotope injection to detect rebleeding. Radionuclide scintigraphy has a false localization rate of approximately 22%, which limits its value as a diagnostic test[11].
Mesenteric angiography can detect bleeding rates greater than 0.5 mL/min and has the advantage of therapeutic intervention through transcatheter embolization. Angiography has a sensitivity of 40%-86%[12,13].
Initial experience indicates that multidetector computed tomography (CT) angiography is a promising first-line modality for the time-efficient, sensitive, and accurate diagnosis or exclusion of active GI hemorrhage and may have a profound impact on the evaluation and subsequent treatment of patients who present with acute GI bleeding.
In this article, we discuss and illustrate the emerging role of CT angiography in the evaluation and localization of acute, active GI hemorrhage.
A comprehensive computer literature search was performed to identify studies assessing the diagnostic value of CT angiography for acute GI bleeding.
The MEDLINE and EMBASE databases, from January 1995 to December 2009, were searched with the following key words: “gastrointestinal hemorrhage” OR “gastrointestinal bleeding” AND “CT angiography” OR “X-ray computed” AND (sensitivity OR specificity OR false-negative OR false-positive OR diagnosis OR detection OR accuracy).
Other databases, such as Sciencedirect, Springerlink, Scopus and the Cochrane library were also checked for relevant articles using the same keywords. The references reported in all retrieved articles were also supplemented with extensive checking.
Two investigators (Wu LM and Xu JR), who were blinded to the journal, author, institution and date of publication, independently checked retrieved articles. According to a standardized data extraction form, we read all the abstracts to obtain potentially eligible articles, and then we managed to get the full text of these articles to determine whether they were exactly eligible. Disagreements were resolved by consensus.
The inclusion criteria were (1) articles were published in English; (2) CT was used as the index test in the diagnosis of GI bleeding; (3) The reference test had to be angiography, endoscopy, colonoscopy, surgery or a combination; (4) For per-patient statistics, sufficient data were presented to calculate the true-positive (TP), false-negative (FN), false-positive (FP) and true-negative (TN) values; (5) 5 or more patients were included; (6) With regard to the quality of the study design, only articles in which the number of “yes” answers for the 14 questions in the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) quality assessment tool[14] was more than 9 were included; and (7) When data or subsets of data were presented in more than one article, the article with most details or the most recent article was chosen. The authors of abstracts and studies not reporting sufficient data were contacted to request additional information.
The excluded criteria were as follows: (1) Articles which did not include raw data such as reviews, case reports, comments, editorials, letters and congress; (2) Articles which used CT as the tool for diagnosis without information on CT alone; (3) Diagnosis of GI bleeding for other existing diseases and could not be differentiated; and (4) Reports specializing in the etiology of bleeding.
A quality assessment tool for diagnostic accuracy studies, named “QUADAS”, was used to evaluate the quality and to extract relevant study design characteristics of included studies[15]. This tool is an evidence-based quality assessment tool developed for use in systematic reviews of studies of diagnostic accuracy and was fully described by Whiting et al[14].
2 × 2 tables were extracted on per-patient basis, including the numbers of TP, TN, FP and FN results in each study. Pooled sensitivity and specificity, with 95% CI, were obtained. A value of 0.5 was added to all cells of studies that contained a count of zero to avoid potential problems in odds calculations for studies with sensitivities or specificities of 100%.
Heterogeneity was assessed with the χ2-test using a random effects model (DerSimonian and Laird). A summary receiver operating characteristic (SROC) curve was fitted using the Moses and Littenberg model and a weighted area under the curve (AUC) obtained to measure the diagnostic performance of CT angiography. Statistical analysis was conducted using META-DISC software (version 1.4; Clinical Biostatistics Unit, Ramo′ny Cajal Hospital, Madrid, Spain).
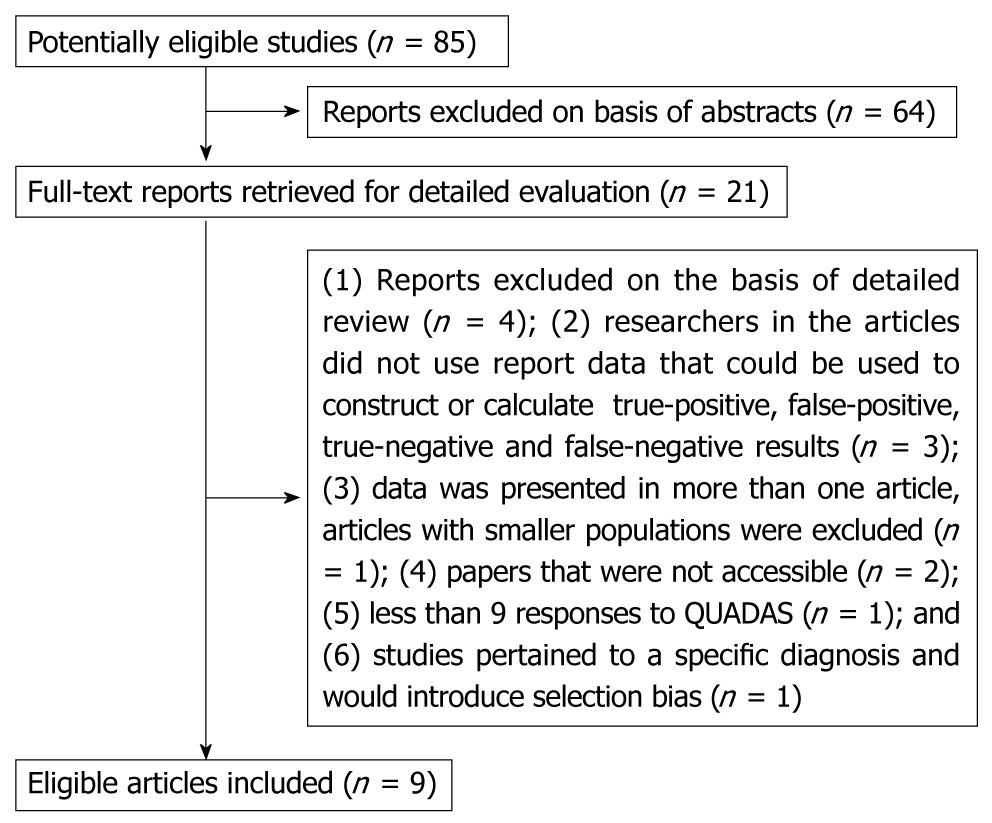
The detailed procedure of study selection for the meta-analysis is shown in Figure 1. Eighty five initial studies were searched from all the databases. After reading the abstracts, we reviewed 21 studies in detail. Of these articles, 12 were excluded because: (1) Reports excluded on the basis of detailed review (n = 4); (2) researchers in the articles did not use report data that could be used to construct or calculate TP, FP, TN and FN results (n = 3); (3) data was presented in more than one article, articles with smaller populations was excluded (n = 1); (4) paper was not accessible (n = 2); (5) less than 9 responses to QUADAS (n = 1); and (6) studies pertained to a specific diagnosis and would introduce selection bias (n = 1).
Finally, 9 articles fulfilled all the inclusion criteria and were selected for data extraction and data analysis. We obtained the full text for all 9 eligible studies, some features of each are shown in Table 1.
| Author | Yr | n | Age (average, yr) | Study design | Patient selection | Type of CT | Reference standard | Male/female | QUADASscore |
| Ettorre et al[16] | 1997 | 18 | N/A | Prospective | Unselected | Single slice | Conventional angiography or surgery | N/A | 9 |
| Ernst et al[17] | 2003 | 24 | 59 (18-85) | Prospective | Unselected | Single slice | Colonoscopy, enteroscopy or surgery | 15/4 | 10 |
| Tew et al[18] | 2004 | 13 | N/A | Retrospective | Unselected | 4-slice | Conventional angiography, surgery or clinical follow-up | 15/4 | 9 |
| Miller et al[19] | 2004 | 18 | 69 (43-83) | Prospective | Selected | Dual slice | Endoscopy, colonoscopy or conventional angiography | 9/9 | 6 |
| Sabharwal et al[20] | 2006 | 7 | 69 (48-83) | Prospective | Selected | 4-slice | Conventional angiography or colonoscopy | 2/5 | 10 |
| Yoon et al[21] | 2006 | 26 | 66 (18-89) | Prospective | Unselected | 4-slice | Digital subtraction angiography | 17/9 | 12 |
| Jaeckle et al[22] | 2008 | 36 | 51 (4-85) | Retrospective | Selected | 16, 40-slice | Endoscopy, or surgery | 22/14 | 10 |
| Zink et al[23] | 2008 | 41 | 55 (21-92) | Prospective | Selected | 16-slice | Labeled red blood cell scan or surgery | N/A | 9 |
| Lee et al[24] | 2009 | 15 | 72 (42-90) | Retrospective | Unselected | 16, 64-slice | Conventional angiogram, colonoscopy, capsule enteroscopy, labeled red blood cell scan or surgery | 9/6 | 9 |
The median number of participants per study was 18 (range 7-41). Our study reported the results by using an individual patient as the unit of analysis. Seven studies used multidetector CT (MDCT), which ranged from a dual-slice CT to a 64-slice CT. Protocols for CT scanning varied among studies, with one study using water as an oral contrast[19], and another study using intraarterial contrast[16]. The criterion for a positive CT was generally extravasation of contrast into the bowel lumen. The study by Ernst et al[17] used a broader definition by also including contrast enhancement of the bowel wall, presence of vascular abnormalities, polyp, tumor or spontaneous hyperdensity of peribowel fat. The reference standard among studies consisted of angiography (either digital subtraction or conventional angiography), upper GI endoscopy, colonoscopy and surgery, either alone or in combination.
Of the 9 studies, six[16,17,19,20-23] enrolled patients prospectively. Three studies[18,22,24] were retrospective database reviews.
The severity of GI bleeding varied and included patients who did not require blood transfusion to patients who required multiple transfusions. One study focused on massive GI bleeding - which was defined as hemodynamic instability or a transfusion requirement of greater than 4 units of packed cells within a 24-h period[21]. Two primary studies pertained to lower GI bleeding exclusively[18,20].
The quality and completeness of the reporting of studies was variable (quality scores in Table 1). Three studies were retrospective in nature and interpretation of the tests was blinded in only two studies. The reference test was not standardized among studies, raising the possibility of differential verification bias affecting estimates of accuracy.
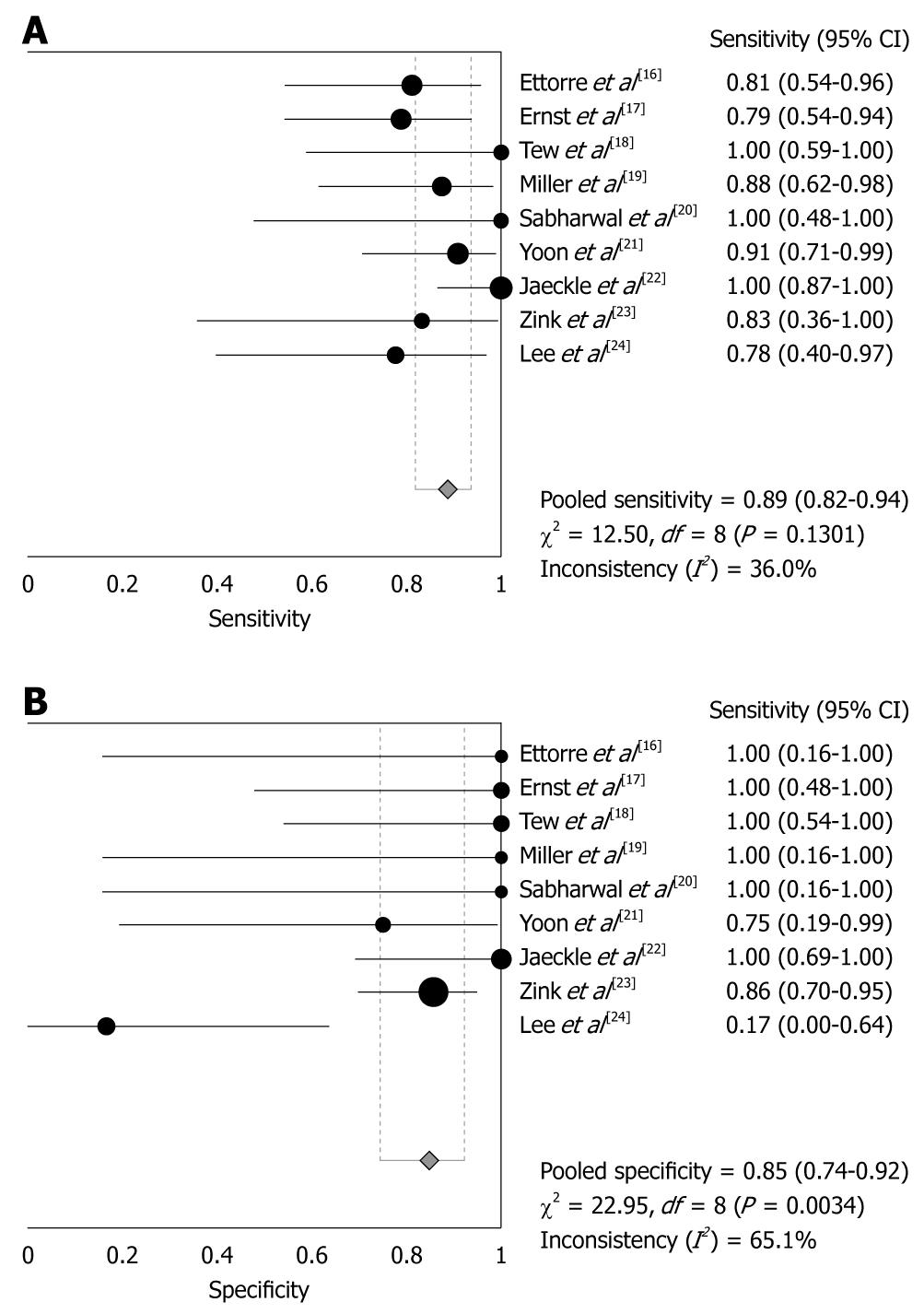
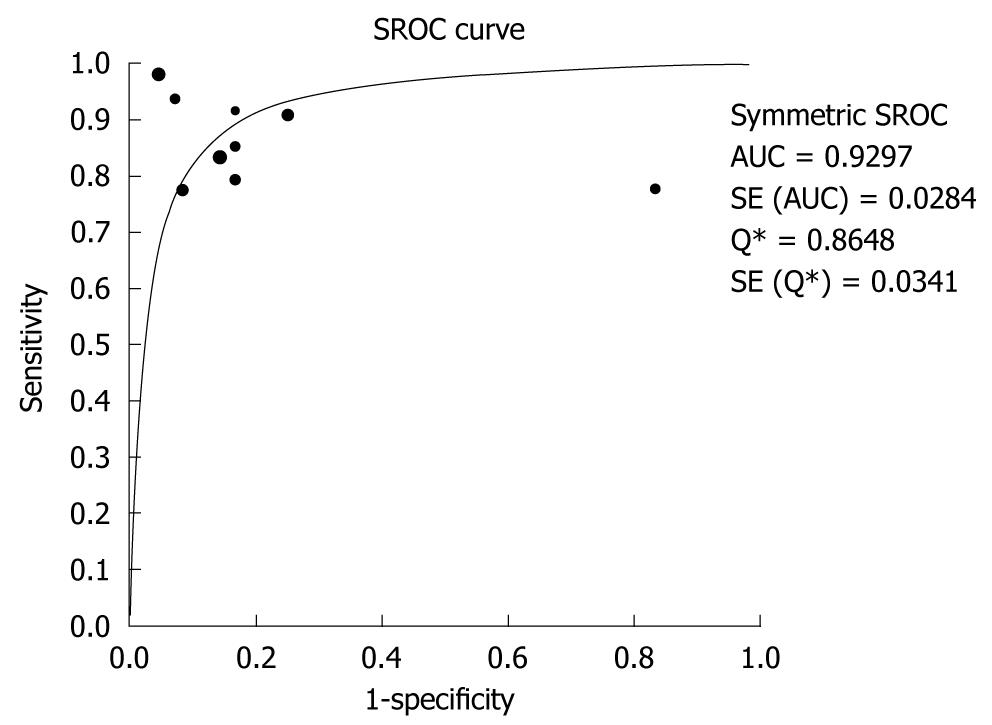
CT angiography showed pooled sensitivity of 89% (95% CI: 82%-94%) without significant heterogeneity (χ2 = 12.5, P = 0.13). Pooled specificity was 85% (95% CI: 74%-92%) without showing significant heterogeneity (χ2 = 22.95, P = 0.003). Using the fitted SROC curve, overall AUC was 0.9297, indicating very good diagnostic accuracy. TP, FN, FP and TN results are shown in Table 2. Sensitivities and specificities are showed in Figure 2, and the SROC curve is shown in Figure 3.
| Study | n | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
| Ettorre et al[16] | 18 | 13 | 0 | 3 | 2 | 0.81 (0.54-0.96) | 1.00 (0.16-1.00) |
| Ernst et al[17] | 24 | 15 | 0 | 4 | 5 | 0.79 (0.54-0.94) | 1.00 (0.48-1.00) |
| Tew et al[18] | 13 | 7 | 0 | 0 | 6 | 1.00 (0.59-1.00) | 1.00 (0.54-1.00) |
| Miller et al[19] | 18 | 14 | 0 | 2 | 2 | 0.88 (0.62-0.98) | 1.00 (0.16-1.00) |
| Sabharwal et al[20] | 7 | 5 | 0 | 0 | 2 | 1.00 (0.48-1.00) | 1.00 (0.16-1.00) |
| Yoon et al[21] | 26 | 20 | 1 | 2 | 3 | 0.91 (0.71-0.99) | 0.75 (0.19-0.99) |
| Jaeckle et al[22] | 36 | 26 | 0 | 0 | 10 | 1.00 (0.87-1.00) | 1.00 (0.69-1.00) |
| Zink et al[23] | 41 | 5 | 5 | 1 | 30 | 0.83 (0.36-1.00) | 0.86 (0.70-0.95) |
| Lee et al[24] | 15 | 7 | 5 | 2 | 1 | 0.78 (0.40-0.97) | 0.17 (0.00-0.64) |
In the study by Miller et al[19], six patients had a source of bleeding suggested on CT that was not detected with endoscopy or surgery. One of these patients had a 99mTc red cell scan, which showed a bleeding site in the cecum. The bleeding sites of the other five patients were attributed to bleeding that subsequently stopped or bleeding from a small bowel source, which was difficult to detect with endoscopy. In the absence of any alternative diagnoses, the five positive CT studies were classified as true positives.
Acute GI bleeding is an emergency situation with high mortality rates. Therefore, fast detection and localization of the bleeding site are required for effective hemostatic therapy. Currently, the first diagnostic procedure in patients with upper GI tract bleeding is endoscopy, whereas colonoscopy, conventional angiography or 99mTc-RBC scintigraphy are the standard procedures in patients with lower GI tract hemorrhage. Unfortunately, effective endoscopy may be hampered by blood clots or feces obscuring the bleeding site in unprepared patients, while potential disadvantages of conventional invasive angiography include an increased risk of complications[13]. Inherent drawbacks of scintigraphy are that it is a time-consuming method with only limited sensitivity for the identification of bleeding sites.
Fortunately, with high sensitivity, as shown in our review, CT angiography is a possible option for the investigation of GI bleeding. In an animal model of colonic hemorrhage, Kuhle et al[25] reported that single detector helical CT angiography can depict active hemorrhage with a rate of 0.3 mL/min, thus exceeding the sensitivity of mesenteric angiography of 0.5 mL/min and approaching values of RBC scintigraphy of 0.2 mL/min.
Due to higher spatial and temporal resolution provided by MDCT technology, faster scanning times and improved multiplanar reformatted images, the limited number of studies available precludes subgroup analysis to assess whether this has improved the diagnostic accuracy of CT.
Numerous studies have been performed to evidence the role of CT relative to conventional angiography for the diagnostic workup of patients suffering from acute GI bleeding[15,25-27]. These reports include case studies by Krestan et al[26], and Singer[27] as well as single-detector row helical CT studies performed in patients[16] and animals[25]. Several studies in our series have shown that CT can diagnose bleeding where angiography failed to locate the source[16,20,28]. In the study by Sabharwal et al[20], CT showed the site of bleeding in three patients for whom conventional angiography was negative. Subsequent emergency colonoscopy confirmed the presence of a blood-filled colon without showing the site of bleeding.
Jaeckle et al[22] evaluated the accuracy of MDCT for detection and localization of acute upper and lower GI hemorrhage or intraperitoneal bleeding. Thirty-six consecutive patients with clinical signs of acute bleeding underwent biphasic MDCT. MDCT findings were correlated with endoscopy, angiography or surgery. Among the 36 patients evaluated, 26 were examined for GI bleeding and 10 for intraperitoneal hemorrhage. Confirmed sites of GI bleeding were the stomach (n = 5), duodenum (n = 5), small bowel (n = 6), large bowel (n = 8) and rectum (n = 2). The correct site of bleeding was identifiable on MDCT in 24/26 patients with GI bleeding.
The sensitivity of CT may be higher than reported because GI bleeding is intermittent in nature and the rate of bleeding can vary from minute to minute[29]. A patient may have active GI bleeding shown on CT, which ceased by the time colonoscopy or angiography was carried out. The article by Miller et al[19] had five cases with a source of bleeding visible on CT that other methods of investigation failed to detect.
CT angiography is an excellent diagnostic tool for fast and accurate detection and localization of acute GI hemorrhage and intraperitoneal bleeding. Advantages of MDCT for the diagnostic workup of patients with suspected acute abdominal hemorrhage include widespread availability, speed, reproducibility and minimal invasiveness. The complications of invasive angiography, such as groin hematoma, dissection and distal embolization are reported to occur in 1.3%-2.2% of procedures[30,31]. Moreover, CT scanning of the abdomen can be performed immediately during hemorrhagic episodes, and bleeding can be depicted within the small bowel, an anatomic region not readily accessible to endoscopy. Furthermore, when a bleeding site is shown on CT and the initial mesenteric angiographic examination is negative, delayed selective angiography can be carried out to locate and embolize the bleeding vessel[32].
Limitations of CT angiography for detection of acute GI bleeding are the lack of therapeutic options in comparison to those that are available with endoscopy, colonoscopy and angiography, radiation dose, and risks affiliated with contrast material such as allergy, nephropathy, or hyperthyreosis. Another disadvantage of CT angiography is that metallic artifacts can interfere with visualization of contrast in the bowel lumen and lead to false positive results on CT angiography, as shown in the study by Yoon et al [33].
In this meta-analysis, bias was considered. To avoid selection bias, not only the MEDLINE and EMBASE databases but also the Sciencedirect, Springerlink, Scopus, and the Cochrane library were searched for relevant articles.
To minimize bias in the selection of studies and in data extraction, reviewers who were blinded to the journal, author, institution and date of publication, independently selected articles on the basis of inclusion criteria. In addition, scores were assigned to study design characteristics and examination results by using a standardized form that was based on the QUADAS tool. The QUADAS tool is an evidence-based quality assessment tool, which was developed for use in systematic reviews of studies of diagnostic accuracy[14].
To ensure all the selected articles were high quality articles, only articles in which the number of “yes” answers for the 14 questions in the QUADAS quality assessment tool[14] was more than 9, were selected. If the number of “no” or “unclear” answers was more than 4, the article was excluded. In this way, we excluded low quality articles to make sure the results of this research are credible.
However, some limitations still exist in this meta-analysis. A potential limitation of any meta-analysis is the possibility of publication bias because studies with optimistic results may be published easier than studies with unfavorable results, and studies with large sample size may be published easier than studies with small sample size. We attempted to examine publication bias by evaluating whether the size of the studies was associated with the results for diagnostic accuracy. No association was found between sample size and diagnostic accuracy.
The inclusion bias should be considered in our study. Only the studies published in English were selected in this meta-analysis. This could cause unavoidable bias. However, this bias was small because most studies of high quality were published in English. Besides, all primary studies had relatively low numbers of subjects (n = 7 to n = 41). In practice, larger numbers of subjects are difficult to obtain because CT angiography is not commonly used as an investigation for GI bleeding at most institutions[33]. Thirdly, the overall quality of included studies, as defined in the QUADAS tool, was only moderate. Finally, clinical heterogeneity among studies is also an issue. The severity of GI bleeding varied, with one study focusing on “massive” GI bleeding. The primary different definitions for a positive CT may have resulted in misclassification.
We propose the routine use of CT angiography in the initial radiological investigation of patients who meet the criteria for acute GI hemorrhage. Because it is accurate in the diagnosis of acute GI bleeding and can show the precise location and etiology of bleeding, thereby directing further management. However, further large prospective studies are needed to define the role of CT in acute GI bleeding when other investigations are unable to provide a diagnosis.
Acute gastrointestinal (GI) bleeding is an emergency situation with high mortality rates. Fast detection and localization of the bleeding site are required for effective hemostatic therapy. Due to higher spatial and temporal resolution provided by multi-row detector computed tomography (MDCT) technology, acquisition of arterial- and portal-venous phase images as well as depiction of active extravasation of contrast material have become feasible.
MDCT is an excellent diagnostic tool for fast and accurate detection and localization of acute GI hemorrhage and intraperitoneal bleeding. Advantages of MDCT for the diagnostic workup of patients with suspected acute abdominal hemorrhage include widespread availability, speed, reproducibility and minimal invasiveness. Moreover, computed tomography (CT) scanning of the abdomen can be performed immediately during hemorrhagic episodes, and bleeding can be depicted within the small bowel, an anatomic region not readily accessible to endoscopy.
Currently, CT angiography is not widely used in the diagnosis of acute GI bleeding. This meta-analysis confirmed that it has the advantage of being a noninvasive test, which diagnoses the site and cause of bleeding, thereby guiding definitive treatment.
Invasive selective interventional angiography or surgery can be performed immediately after MDCT has identified the bleeding site. This diagnostic algorithm may lead to a significant reduction in time and may substantially decrease the rate of negative angiographies in the future.
This is a systematic review concerning the usefulness of CT angiography on acute GI bleeding. Authors have nicely performed the meta-analysis for the “usefulness of CT angiography in diagnosing acute GI bleeding”. This article is well written and beneficial for many physicians.
| 1. | Manning-Dimmitt LL, Dimmitt SG, Wilson GR. Diagnosis of gastrointestinal bleeding in adults. Am Fam Physician. 2005;71:1339-1346. |
| 2. | Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843-857. |
| 3. | Imdahl A. Genesis and pathophysiology of lower gastrointestinal bleeding. Langenbecks Arch Surg. 2001;386:1-7. |
| 4. | Lee EW, Laberge JM. Differential diagnosis of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7:112-122. |
| 5. | Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92:419-424. |
| 6. | Junquera F, Quiroga S, Saperas E, Pérez-Lafuente M, Videla S, Alvarez-Castells A, Miró JR, Malagelada JR. Accuracy of helical computed tomographic angiography for the diagnosis of colonic angiodysplasia. Gastroenterology. 2000;119:293-299. |
| 7. | Hara AK, Leighton JA, Sharma VK, Heigh RI, Fleischer DE. Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiographics. 2005;25:697-711; discussion 711-718. |
| 8. | Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6:637-646. |
| 9. | Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol. 2009;12:80-91. |
| 10. | Zuckier LS. Acute gastrointestinal bleeding. Semin Nucl Med. 2003;33:297-311. |
| 11. | Fallah MA, Prakash C, Edmundowicz S. Acute gastrointestinal bleeding. Med Clin North Am. 2000;84:1183-1208. |
| 12. | Vernava AM 3rd, Moore BA, Longo WE, Johnson FE. Lower gastrointestinal bleeding. Dis Colon Rectum. 1997;40:846-858. |
| 13. | Cohn SM, Moller BA, Zieg PM, Milner KA, Angood PB. Angiography for preoperative evaluation in patients with lower gastrointestinal bleeding: are the benefits worth the risks? Arch Surg. 1998;133:50-55. |
| 14. | Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. |
| 15. | Liu T, Xu W, Yan WL, Ye M, Bai YR, Huang G. FDG-PET, CT, MRI for diagnosis of local residual or recurrent nasopharyngeal carcinoma, which one is the best? A systematic review. Radiother Oncol. 2007;85:327-335. |
| 16. | Ettorre GC, Francioso G, Garribba AP, Fracella MR, Greco A, Farchi G. Helical CT angiography in gastrointestinal bleeding of obscure origin. AJR Am J Roentgenol. 1997;168:727-731. |
| 17. | Ernst O, Bulois P, Saint-Drenant S, Leroy C, Paris JC, Sergent G. Helical CT in acute lower gastrointestinal bleeding. Eur Radiol. 2003;13:114-117. |
| 18. | Tew K, Davies RP, Jadun CK, Kew J. MDCT of acute lower gastrointestinal bleeding. AJR Am J Roentgenol. 2004;182:427-430. |
| 19. | Miller FH, Hwang CM. An initial experience: using helical CT imaging to detect obscure gastrointestinal bleeding. Clin Imaging. 2004;28:245-251. |
| 20. | Sabharwal R, Vladica P, Chou R, Law WP. Helical CT in the diagnosis of acute lower gastrointestinal haemorrhage. Eur J Radiol. 2006;58:273-279. |
| 21. | Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239:160-167. |
| 22. | Jaeckle T, Stuber G, Hoffmann MH, Jeltsch M, Schmitz BL, Aschoff AJ. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol. 2008;18:1406-1413. |
| 23. | Zink SI, Ohki SK, Stein B, Zambuto DA, Rosenberg RJ, Choi JJ, Tubbs DS. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR. Am J Roentgenol. 2008;191:1107-1114. |
| 24. | Lee S, Welman CJ, Ramsay D. Investigation of acute lower gastrointestinal bleeding with 16- and 64-slice multidetector CT. J Med Imaging Radiat Oncol. 2009;53:56-63. |
| 25. | Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228:743-752. |
| 26. | Krestan CR, Pokieser P, Wenzl E, Leitha T. Localization of gastrointestinal bleeding with contrast-enhanced helical CT. AJR Am J Roentgenol. 2000;174:265-266. |
| 27. | Singer AA. Value of CT in localizing site of gastrointestinal hemorrhage following negative angiography. Abdom Imaging. 1995;20:31-32. |
| 28. | Hyare H, Desigan S, Nicholl H, Guiney MJ, Brookes JA, Lees WR. Multi-section CT angiography compared with digital subtraction angiography in diagnosing major arterial hemorrhage in inflammatory pancreatic disease. Eur J Radiol. 2006;59:295-300. |
| 29. | Sos TA, Lee JG, Wixson D, Sniderman KW. Intermittent bleeding from minute to minute in acute massive gastrointestinal hemorrhage: arteriographic demonstration. AJR Am J Roentgenol. 1978;131:1015-1017. |
| 30. | Pennoyer WP, Vignati PV, Cohen JL. Management of angiogram positive lower gastrointestinal hemorrhage: long term follow-up of non-operative treatments. Int J Colorectal Dis. 1996;11:279-282. |
| 31. | Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology. 1992;182:243-246. |
| 32. | Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol. Clin North Am. 2005;34:679-698. |
| 33. | Yoon W, Jeong YY, Kim JK. Acute gastrointestinal bleeding: contrast-enhanced MDCT. Abdom Imaging. 2006;31:1-8. |
Peer reviewers: Dr. Ram Prakash Galwa, MBBS, MD, Department of Diagnostic Imaging, The Ottawa Hospital, 751 Parkdale Avenue, Apartment 803, Ottawa, k1y1j7, Canada; Itaru Endo, MD, PhD, Professor and Chairman, Department of Gastroenterological Surgery, Yokohama City University, Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama 2360004, Japan; Serdar Karakose, Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM