Published online Mar 21, 2010. doi: 10.3748/wjg.v16.i11.1397
Revised: November 12, 2009
Accepted: November 19, 2009
Published online: March 21, 2010
AIM: To evaluate the prognostic value of the combined model for end-stage liver disease (MELD) and blood lipid level in patients with decompensated cirrhosis.
METHODS: A total of 198 patients with decompensated cirrhosis were enrolled into the study. The values of triglyceride (TG), cholesterol (TC), high density lipoproteins (HDL) and low density lipoprotein (LDL) of each patient on the first day of admission were retrieved from the medical records, and MELD was calculated. All the patients were followed up for 1 year. The relationship between the change of blood lipid level and the value of MELD score was studied by analysis of variance. The prognostic factors were screened by multivariate Cox proportional hazard model. Draw Kaplan-Meier survival curves were drawn.
RESULTS: Forty-five patients died within 3 mo and 83 patients died within 1 year. The levels of TG, TC, HDL and LDL of the death group were all lower than those of the survivors. The serum TG, TC, HDL and LDL levels were lowered with the increase of the MELD score. Multivariate Cox proportional hazard model showed that MELD ≥ 18 and TC ≤ 2.8 mmol/L were independent risk factors for prognosis of decompensated cirrhosis. Survival analysis showed that MELD ≥ 18 combined with TC ≤ 2.8 mmol/L can clearly discriminate between the patients who would survive and die in 1 year.
CONCLUSION: MELD ≥ 18 and TC ≤ 2.8 mmol/L are two important indexes to predict the prognosis of patients with decompensated cirrhosis. Their combination can effectively predict the long-term prognosis of patients with decompensated cirrhosis.
- Citation: Jiang M, Liu F, Xiong WJ, Zhong L, Xu W, Xu F, Liu YB. Combined MELD and blood lipid level in evaluating the prognosis of decompensated cirrhosis. World J Gastroenterol 2010; 16(11): 1397-1401
- URL: https://www.wjgnet.com/1007-9327/full/v16/i11/1397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i11.1397
Cirrhosis is a common disease and the major cause of death in China. There are liver function failure, portal hypertension and a variety of complications during decompensated period, leading to a high mortality. The true and objective judgments on end-stage liver disease conditions are helpful for clinicians to choose appropriate treatment protocols for individual patients. With continuous emergence of new treatments for liver diseases in recent years, the evaluation system used for the severity and prognosis of patients with decompensated cirrhosis is needed urgently. Model for end-stage liver disease (MELD) established in recent years is a new method to determine the reserved liver function. It has shown many advantages in clinical use, especially in liver transplantation[1,2]. But the model does not contain all prognostic factors, thus it has become a hotspot in the studies to improve the predictive power of MELD score by combination with other evaluation indexes[3-5]. The liver is the vital organ to synthesize blood lipid and lipoprotein. Whether it is injured seriously or otherwise determines the level of blood lipids and apolipoprotein to a large extent. Some papers have shown that the lipid level was an important indicator to reflect the liver damage, which can be used to determine the condition and estimate the prognosis of the patients[6]. In this study, we observed the value of four indexes of triglyceride (TG), cholesterol (TC), high density lipoproteins (HDL) and low density lipoprotein (LDL) to judge the prognosis of patients with decompensated cirrhosis. We also observed the value of combined MELD and TC in evaluating the prognosis of patients with decompensated cirrhosis.
A total of 198 patients with decompensated cirrhosis treated in Department of Gastroenterology of Shanghai East Hospital from October 2005 to June 2008 were evaluated, and their medical profiles were retrospectively analyzed. The clinical diagnoses were all based on the program of 2000 for the prevention and treatment of virus hepatitis established in Xi’an congress[7]. We excluded patients with past or current hepatocellular carcinoma, diseases affecting blood lipid such as hypertension, diabetes, cardiovascular and cerebrovascular disease, kidney disease and so on, use of lipid-regulating drugs recently, admission to hospital repeatedly, incomplete case records and lost to follow-up. The patients aged 26-88 years (61.4 ± 10.5 years), including 122 (61.6%) men and 76 (38.4%) women.
Baseline laboratory results of all the patients obtained at admission (i.e. bilirubin, creatinine, INR, TG, TC, HDL and LDL) were retrieved from the medical records. All the patients were followed up for 1 year. The outcome was assessed based on the 3- and 12-mo mortalities.
All the prognostic models were calculated based on laboratory results obtained on the first day of admission. The MELD equation was used to calculate the severity score: 9.6 × loge [creatinine (mg/dL)] + 3.8 × loge [bilirubin (mg/dL)] + 11.2 × loge (INR) + 6.43[8].
All statistical analyses were conducted with the SPSS for Windows version 13 release. The measurement data between two sets were compared by Student’s t test. The data between multiple sets were compared by analysis of variance. The factors affecting the prognosis were screened by the Cox proportional hazards model. The survival curve was established by Kaplan-Meier analysis and compared by Log rank test. P < 0.05 was accepted as significant.
Forty-five patients died (22.7%) at 3 mo and 83 patients died at 1 year (41.9%) among the 198 patients with cirrhosis. The scores of MELD (23.3 ± 9.82), TG (0.80 ± 0.29 mmol/L), TC (2.63 ± 1.04 mmol/L), HDL (0.93 ± 0.46 mmol/L) and LDL (1.67 ± 0.77 mmol/L) in the death group at 3 mo were significantly different statistically compared with those in the survival group (13.6 ± 6.40, 0.86 ± 0.36 mmol/L, 3.22 ± 1.07 mmol/L, 1.30 ± 0.60 mmol/L and 1.88 ± 0.71 mmol/L) (Table 1). The scores of MELD (22.90 ± 8.90), TG (0.76 ± 0.26 mmol/L), TC (2.77 ± 1.00 mmol/L), HDL (1.06 ± 0.62 mmol/L) and LDL (1.66 ± 0.69 mmol/L) of the death group at 1 year were also significantly different statistically from the survival group (12.42 ± 5.29, 0.94 ± 0.39 mmol/L, 3.36 ± 1.11 mmol/L, 0.93 ± 0.46 mmol/L and 1.67 ± 0.77 mmol/L) (Table 1).
| Clinical features | Follow-up at 3 mo | Follow-up at 1 yr | ||||
| Survival group | Death group | P value | Survival group | Death group | P value | |
| MELD | 13.6 ± 6.40 | 23.3 ± 9.82 | 0.000a | 12.42 ± 5.29 | 22.90 ± 8.90 | 0.000a |
| TG (mmol/L) | 0.86 ± 0.36 | 0.80 ± 0.29 | 0.036a | 0.94 ± 0.39 | 0.76 ± 0.26 | 0.003a |
| TC (mmol/L) | 3.22 ± 1.07 | 2.63 ± 1.04 | 0.006a | 3.36 ± 1.11 | 2.77 ± 1.00 | 0.001a |
| HDL (mmol/L) | 1.30 ± 0.60 | 0.93 ± 0.46 | 0.013a | 1.37 ± 0.50 | 1.06 ± 0.62 | 0.021a |
| LDL (mmol/L) | 1.88 ± 0.71 | 1.67 ± 0.77 | 0.010a | 2.04 ± 0.74 | 1.66 ± 0.69 | 0.020a |
The 198 cases of cirrhosis were divided into three groups according to MELD score: < 11 (group 1), 11 ≤ or < 21 (group 2), and ≥ 21 (group 3). The levels of TG, TC, HDL and LDL declined with increasing MELD score. The TC and HDL were all statistically significant among the three groups (Table 2). There was no statistically significant difference in the level TG and LDL between group 1 and group 2, but between group 2 and group 3 (Table 2).
| Group 1 (MELD < 11) | Group 2 (11 ≤MELD < 21) | P value (groups 1 and 2) | Group 3 (MELD≥21) | P value (groups 2 and 3) | |
| TG (mmol/L) | 1.01 ± 0.46 | 0.96 ± 0.33 | 0.089 | 0.80 ± 0.31 | 0.021b |
| TC (mmol/L) | 3.68 ± 1.23 | 3.03 ± 0.91 | 0.006d | 2.62 ± 1.18 | 0.002d |
| HDL (mmol/L) | 1.97 ± 0.73 | 1.34 ± 0.83 | 0.026d | 0.76 ± 0.55 | 0.018d |
| LDL (mmol/L) | 1.89 ± 0.44 | 1.71 ± 0.44 | 0.092 | 1.42 ± 0.86 | 0.032b |
The prognostic factors (MELD ≥ 21, TG ≤ 0.8 mmol/L, TC ≤ 2.8 mmol/L, HDL ≤ 1.2 mmol/L and LDL ≤ 1.6 mmol/L) of 198 patients with cirrhosis were included into the multivariate Cox proportional hazard model for regression analysis. As a result, MELD ≥ 21 and TC ≤ 2.8 mmol/L were independent risk factors for prognosis of decompensated cirrhosis. The risk ratios (HR) were 2.69 and 0.68 (Table 3).
| HR | 95% CI | P | |
| MELD ≥ 21 | 2.69 | 1.58-4.58 | < 0.001 |
| TC ≤ 2.8 mmol/L | 0.68 | 0.31-1.43 | 0.020 |
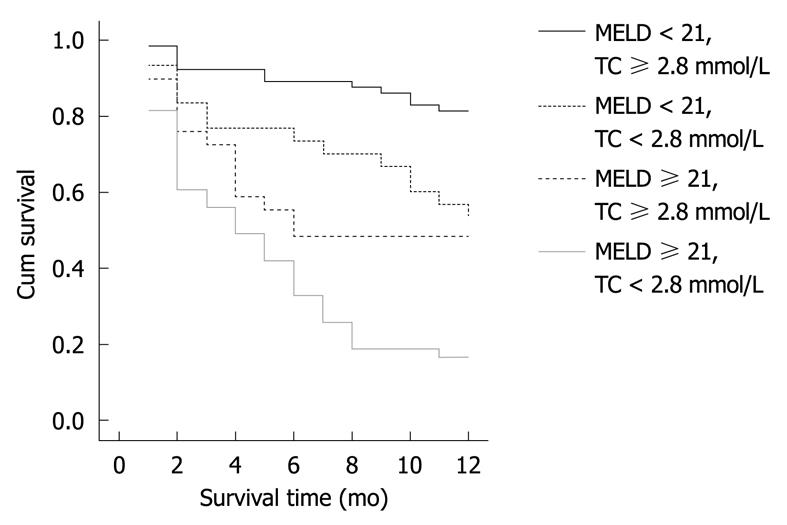
Based on the results of multivariate Cox regression analysis, the MELD ≥ 21 combined with TC ≤ 2.8 mmol/L was divided into four groups. The 1-year survival curve was set up by Kaplan-Meier (Figure 1). It showed that MELD ≥ 21 combined with TC ≤ 2.8 mmol/L can distinguish between the patients who would survive and die in 1 year (Figure 1).
MELD was created by Malinchoc et al[9] of Mayo Clinic, America in 2000, which was initially used to predict survival following elective placement of transjugular intrahepatic portosystemic shunt to prevent bleeding and cure ascites. Later, MELD score has been proven to effectively reflect the severity of pathogenetic condition and prognosis status of different liver diseases, and it has become the recognized standard to judge the severity of pathogenetic condition for end-stage liver disease. Many studies have reported that MELD was indeed a good indicator in determining the condition of end-stage liver disease such as cirrhosis, hepatitis, liver transplantation[1,10]. However, Angermayr et al[11] thought that the judging ability of MELD is not superior to CTP score. Earlier reports confirmed that the MELD scoring system had an advantage in assessment of liver transplanted patients, but recent studies showed that the system was still not perfect, which is not suitable for all patients with liver transplantation[12,13].
MELD score is still controversial for its ability to determine the pathogenetic condition of end-stage liver disease. Currently, scholars are combining MELD with other indicators in an attempt to estimate pathogenetic condition of liver diseases more accurately[14]. The liver is the main site to synthesize, store, transport and decompose lipids, including synthesis and secretion of endogenous lipoprotein; synthesis of rate-limiting enzyme of lipoprotein metabolism to regulate mutual conversion and metabolism between a variety of lipoprotein; and absorbing and removing metabolites of lipoprotein through the lipoprotein receptors on the hepatocytes surface to maintain equilibrium of TC and TG metabolism[15]. The liver cannot esterify fatty acid to synthesize phosphatidyl choline required by TG and TC esterification. The reduced synthesis of TC acyltransferase can decrease the serum TG and TC levels. In addition, damaged hepatocytes, abnormal synthesis of apolipoprotein A by liver and/or the reduced synthesis of phosphatidylcholine TC acyltransferase may decrease the levels of high-density and very low-density lipoproteins. It has been reported that the Child-Pugh score of patients with cirrhosis was high, the higher severity of the liver damage, the lower the blood lipid level, which is an important indicator to reflect liver damage. The blood lipid level can be used to determine the condition and estimate the prognosis of the patients[16]. In this paper, the patients with cirrhosis were divided into low-risk, medium-risk and high-risk groups according to the MELD score. It was also observed that the blood lipid levels in the patients were decreased with increasing MELD score. There was no significant difference in the two indicators (TG and LDL) for cirrhosis between low-risk and medium-risk groups. Considering that extrahepatic tissues of patients may still have some degree of synthesizing ability in early stage of cirrhosis,and there is blood lipid elimination disorder in the cirrhotic patients, it is not likely for blood lipid level to reduce. But with a further reduction in cytoactivity of hepatocytes, blood lipid will significantly reduce. Habib et al[6] have studied 248 cases of cirrhosis and found that the HDL, TC and VLDL level of deaths in 90, 180, 365 d were all lower than that of the survivals. This result also showed that all serum lipids of the patients who died within 3 mo and 1 year were all significantly lower than that of survivals.
The liver plays a leading role in the synthesis of TC and maintain the balance in TC metabolism. It can control the dynamic equilibrium of TC in vivo through participation in the process of uptake, synthesis, intracellular transport, esterification or hydrolysis of TC, and rotation to the circulating blood, and synthesizing and secreting bile acid. TG oxidation (hydrolysis) is inhibited in patients with hepatitis. On the other hand, due to appetite loss and short-term starvation, the liver metabolism mobilized a large number of stored fat, which can raise the TG levels. The liver function was severely damaged during cirrhosis. The hepatocytes lack of energy result in fatty acid oxidation and TG levels decrease. The liver is not only the organ that synthesizes most TC in human body by up to 60%-80%, but also the site for TC transformation and excretion. In liver disease, the level of serum TC is reduced because of decreased TC synthesis in the liver and blocked esterification. With deterioration of liver cell damage, ALT level increased and the serum TC further decreased. Liver function is seriously and irreversibly damaged during cirrhosis, resulting in significant decrease of TC level. So the level of serum TC is meaningful to determine the severity of acute liver disease, which can reflect the acute processes, it is particularly helpful in severe hepatitis judgment. Selcuk et al[17] analyzed 99 dead patients with cirrhosis and found that their TC levels were all lower than normal. Li et al[18] selected six indicators including TC by Logistic regression analysis as the prognostic indicator of patients with chronic severe hepatitis. In our study, MELD ≥ 21 and TC ≤ 2.8 mmol/L were selected by multivariate Cox proportional hazard model as the independent prognostic factors of patients with cirrhosis. HR was 2.69 and 0.68. MELD and the TC values are corresponding to the severity of illness. Kaplan-Meie survival curve showed that MELD ≥ 21 combined with TC ≤ 2.8 mmol/L can distinguish between the patients who would survive and die in 1 year, which may provide more information in clinical treatment. In addition, 90% of the HDL in vivo is synthesized by the liver. The change in serum HDL concentration is closely related to the severity of the liver disease. The progressive reduction of HDL is the performance indicating deteriorated condition, while the level of HDL would gradually increase with the condition improved. Thus, serum HDL is the ideal target for liver disease diagnosis and prognostic evaluation[19]. Habib et al[6] found that HDL was the marker of liver function and prognostic factor of nonalcoholic cirrhosis. After liver transplantation, the mortality of patients was significantly correlated with decreased serum HDL. Especially the risk of death in cirrhotic patients increased by 3.4% as the serum HDL became lower than 30 mg/dL. However, we did not find that HDL is an independent prognostic factor in cirrhotic patients by Cox regression mode. Larger series of patients is needed to verify the findings.
Cirrhosis is a common disease and the major cause of death in China. The true and objective judgments on end-stage liver disease conditions is helpful for clinicians to choose different treatment protocols suitable for individual patients.
Model for end-stage liver disease (MELD) established in recent years is a new method to determine reserved liver function. It has shown many advantages in clinical use, especially in liver transplantation. But the model is not perfect. It is a hotspot in the studies to improve the predictive power of MELD score by combination with other evaluation indexes. This study has shown that the lipid level is an important indicator to reflect the degree of liver damage, which can be used to determine the condition and estimate the prognosis of the patients.
In some studies, the lipid level has been used to determine the condition and estimate the prognosis of patients with liver damage. In this study, the authors observed four indexes of triglyceride, cholesterol (TC), high density lipoproteins and low density lipoprotein to judge the prognosis of 198 patients with decompensated cirrhosis through retrospective analysis, and observed the value of MELD combined with TC in evaluating the prognosis of patients with decompensated cirrhosis as well.
MELD combined with cholesterol can be used to judge the pathogenetic condition and prognosis of patients with decompensated cirrhosis.
Multivariate Cox proportional hazard model is a multivariate technique for analyzing the effect of two or more metric and/or nonmetric variables on survival.
The aims of this paper are to see whether modification of MELD can improve accuracy. The rationale for use of lipids needs to be strengthened. The authors should consider using some of the modifications of MELD such as MELD-Na.
| 1. | Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-358. |
| 2. | Ahmad J, Downey KK, Akoad M, Cacciarelli TV. Impact of the MELD score on waiting time and disease severity in liver transplantation in United States veterans. Liver Transpl. 2007;13:1564-1569. |
| 3. | Wang YW, Huo TI, Yang YY, Hou MC, Lee PC, Lin HC, Lee FY, Chi CW, Lee SD. Correlation and comparison of the model for end-stage liver disease, portal pressure, and serum sodium for outcome prediction in patients with liver cirrhosis. J Clin Gastroenterol. 2007;41:706-712. |
| 4. | Kalabay L, Gráf L, Vörös K, Jakab L, Benko Z, Telegdy L, Fekete B, Prohászka Z, Füst G. Human serum fetuin A/alpha2HS-glycoprotein level is associated with long-term survival in patients with alcoholic liver cirrhosis, comparison with the Child-Pugh and MELD scores. BMC Gastroenterol. 2007;7:15. |
| 5. | Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174-1180. |
| 6. | Habib A, Mihas AA, Abou-Assi SG, Williams LM, Gavis E, Pandak WM, Heuman DM. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin Gastroenterol Hepatol. 2005;3:286-291. |
| 7. | Chinese Society of Infectious Diseases and Parasitology and Chinese Society of Hepatology of Chinese Medical Association. The programme of prevention and cure for viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 8. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. |
| 9. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. |
| 10. | Ahmad J, Downey KK, Akoad M, Cacciarelli TV. Impact of the MELD score on waiting time and disease severity in liver transplantation in United States veterans. Liver Transpl. 2007;13:1564-1569. |
| 11. | Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879-885. |
| 12. | Coombes JM, Trotter JF. Development of the allocation system for deceased donor liver transplantation. Clin Med Res. 2005;3:87-92. |
| 14. | Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-810. |
| 15. | Zeng MD, Xiao SD. Liver and endocrine. Beijing: People's Medicial Publishing House 1997; 119-127. |
| 16. | Guo YY, Yang JH. [The clinical significant of variation of lipid and lipoprotein in patients with hepatocirrhosis]. Linchuang Xiaohuabing Zazhi. 2001;13:120-121. |
| 17. | Selcuk H, Uruc I, Temel MA, Ocal S, Huddam B, Korkmaz M, Unal H, Kanbay M, Savas N, Gur G. Factors prognostic of survival in patients awaiting liver transplantation for end-stage liver disease. Dig Dis Sci. 2007;52:3217-3223. |
| 18. | Li Q, Yuan GY, Tang KC, Liu GW, Wang R, Cao WK. Prognostic factors for chronic severe hepatitis and construction of a prognostic model. Hepatobiliary Pancreat Dis Int. 2008;7:40-44. |
| 19. | Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217-226. |
Peer reviewer: James Neuberger, Professor, Liver Unit, Queen Elizabeth Hospital, Birmingham B15 2TH, United Kingdom
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM