Published online Feb 28, 2009. doi: 10.3748/wjg.15.1014
Revised: February 1, 2009
Accepted: February 8, 2009
Published online: February 28, 2009
We report a case of a 62-year old woman admitted to our hospital for multiple nodular metastatic liver lesions found by ultrasonography in a regular medical examination. Routine laboratory tests were normal. PET-CT showed multiple bone lesions and nodular liver lesions. Liver biopsy revealed nodular infiltration of multiple myeloma with positive staining of kappa light chain. Further investigation of bone marrow aspiration, immunofixation and immunoelectrophoresis of serum protein, urine test for Bence-Jones protein, β2-microglobulin in serum and urine confirmed the diagnosis. The patient also coinfected with hepatitis C virus (HCV). With six cycles of chemotherapy with VAD schedule, she achieved complete remission. In this report, a literature review of liver lesions involving multiple myeloma is also provided.
- Citation: Wu XN, Zhao XY, Jia JD. Nodular liver lesions involving multiple myeloma: A case report and literature review. World J Gastroenterol 2009; 15(8): 1014-1017
- URL: https://www.wjgnet.com/1007-9327/full/v15/i8/1014.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1014
Multiple myeloma (MM) is a malignant plasma cell disorder characterized by plasma cell infiltration of the bone marrow and overproduction of immunoglobulin or light chains. Although plasma cell infiltration of the liver occurs in 40% cases of MM[1], most of them are diffused infiltration and usually reported as autopsy findings. Nodular liver lesions involving MM is a rare condition. We present a case of hepatic nodular lesions identified to be MM by liver biopsy.
A 62-year old woman was admitted to our hospital for multiple nodular metastatic liver lesions found by ultrasonography in a regular medical examination. She had low back pain for four months, which was diagnosed as lumbar vertebral osteoarthrosis, and treated with Votalins, but did not remit. She had thrombocytopenic purpura 20 years ago and received blood transfusion during choledocholithotomy for bile duct stone 10 years ago.
On physical examination, the only positive sign was tenderness in processus spinalis L3-S1 and paravertebral area.
Laboratory tests on admission showed 102 g/L hemoglobin, 5.35 × 109/L white blood cells, 242 × 109/L platelets, 93 mm/h erythrocyte sedimentation rate (ESR). C-reactive protein, liver function test, lactate dehydrogenase (LDH), electrolytes, glucose, fat, and renal function tests were all normal. Prothrombin time (PT) was 12.9 s, prothrombin activity (PTA) was 85.8%. Serum markers for hepatitis B were negative, while hepatitis C antibody was positive, and HCVRNA was 1.47 × 105 IU/L.
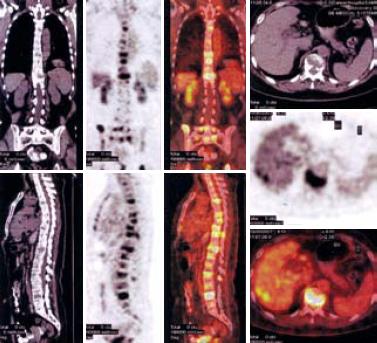
Abdominal computed tomography (CT) showed multiple metastatic space-occupying lesions of the liver and destruction of multiple lumbar vertebral bodies. F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT imaging demonstrated multiple osteolytic bone lesions including vertebrae of cervical, thoracic, lumbar and sacral, and skull, mandible, right humerus, scapula, left clavicle, sternum, ribs, and pelvic bones as well as several hypermetabolic areas in liver, indicating metastatic tumors (Figure 1).
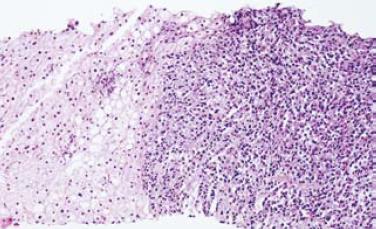
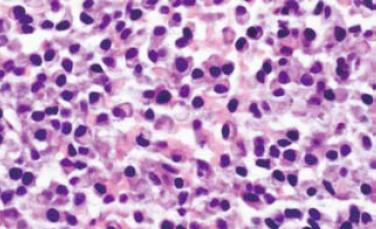
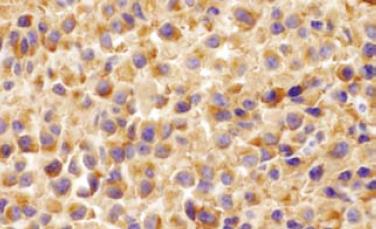
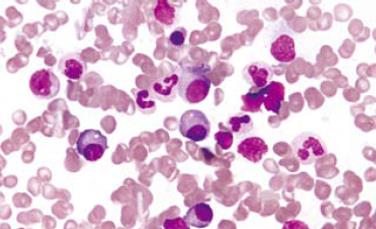
Gastroentestinal endoscopy revealed no primary tumors. Fine-needle liver biopsy under ultrasonography guidance was performed, showing focal lesions of plasma-cell myeloma (Figure 2 and Figure 3), which was positive for immunohistochemistry staining of kappa light chain (Figure 4). Congo red staining showed slight amyloid substance deposition in the sinusoidal area. Histopathologic examination of the right iliac crest confirmed the diagnosis of multiple myeloma (Figure 5). Plasmacytosis (28.5%) showed immature plasmablasts with prominent nucleoli. The number of residual normal hemopoeitic cells was markedly reduced.
The hallmark of MM is the detection of monoclonal and M protein in blood and/or urine, produced by abnormal plasma cells. Immunofixation and immunoelectrophoresis confirmed the presence of a broad monoclonal band serum protein in β globulin regions. The band type was identified as IgA kappa at a concentration of 1770 mg/dL. Urine test for Bence-Jones protein was positive. β2-microglobulin in serum and urine were 3.71 &mgr;g/mL (reference range 1.17-2.29) and > 8 mg/24 h (reference range 0-0.637), respectively. Creatinine clearance rate was 54.8 mL/min (reference range 75-115). Finally, the patient was diagnosed as multiple myeloma, IgA kappa light chain, grade II, with nodular liver involvement, and coinfection with hepatitis C virus (HCV).
She started systemic chemotherapy with Bortezomib, vincristine, doxorubicin along with oral dexamethasone, and achieved complete remission. Bone marrow aspiration showed that the number of plasma cells was less than 1%. Immunofixation and immunoelectrophoresis detected no monoclonal band serum protein. Urine Bence-Jones protein was negative. β2-microglobulin in serum and urine decreased to 2.2 &mgr;g/mL and 0.857 mg/24 h, respectively. CT scan of the abdomen six months after therapy showed no focal lesions in liver and SPECT did not find new lesions in bone.
Nodular liver lesion has been proved to be MM by liver biopsy, which is a clonal B-lymphocyte neoplasm of terminally differentiated plasma cells. Recurrent bacterial infection, anemia, osteolytic lesions and renal insufficiency are the most common clinical features of MM. Our patient had a history of low back pain for four months, but her uncharacterized laboratory tests and image examination did not show enough evidence of MM at the beginning. Then, metastatic nodular liver lesions were incidentally found during a regular medical examination. From a clinical point of view, metastatic liver lesions might have led us to think of metastasis of primary adenocarcinoma from gastrointestinal tract, breast, and adrenal glands. Unexpectedly, fine-needle aspiration showed nodular plasma-cell myeloma in liver. PET-CT scan showed characteristic multiple liver lesions and many skeletoles, but no other organ infiltration.
When MM affects the liver, diffused (sinusoidal, portal, mixed) and nodular patterns of microscopic plasma cell infiltration have been described[1]. Diffused rather than nodular hepatic infiltration is the predominant pattern of liver involvement. Perez-Soler et al[2] reported 128 patients with MM, histologic findings were available in the liver of 21 patients, and diffuse plasma cell infiltration of the liver was observed in 10 patients, while no case of nodular liver infiltration was observed. In a database of 2584 patients with MM[3], liver involvement could be found as masses or macroscopic nodules only in 9 cases. However, in a retrospective study on 52 autopsied cases with MM[4], hepatic invasion can be observed in 15 patients (28.8%) with circumscribed tumor nodules in 7 cases (13.4%) and diffuse tumor involvement in 8 cases (15.4%). In fact, most nodular liver lesions involving MM have been reported in single cases[5–9].
Myeloma cells proliferate in bone marrow and circulate through bloodstream. Like benign plasma cells, they circulate through lymphatics and the reticuloendothelial system. Hence spleen, liver or lymph node infiltrations are common. Amyloidosis and myeloid metaplasia are also histological changes leading to clinical presentations. Liver lesions involving MM may present with hepatomegaly, jaundice, ascites, fulmnant liver failure, or are totally asymptomatic incidentally found by autopsy or image examination. It was reported[1] hepatolmegaly can be found in 58% cases of MM and plasma cell infiltration of the liver is confirmed in 40% cases of MM. However, in all hematological malignancies[10], liver involvement is found in 32% of cases of myeloma, 80%-100% in chronic leukemia and myeoproliferative diseases, 60%-70% in acute leukemia, and 50%-60% in non-Hodgkin’s lymphoma.
The correlation between paraprotein type and incidence of extraosseous tumor in patients with myeloma still remains unclear[11]. Edward et al[12] suggested that extraosseous involvement may be more prominent in patients with IgA myeloma. Oshima et al[4] found that extraosseous involvement of the liver is more frequent in IgA myeloma than in other types of myeloma, although the reason is unknown. In our case, IgA kappa light chain deposited in the liver sinus was confirmed by immunohistochemistry.
Interestingly, our patient was also coinfected with HCV. HCV is lymphotropic and may replicate in lymphocytes and hepatocytes. It was reported that multiple myelomas may develop in a patient with chronic hepatitis C[13]. The prevalence of HCV infection in patients with MM is 6.5%[14]. In contrast to HBV exacerbation, HCV rarely causes fulminant hepatitis, but less is known about the exacerbation of chronic HCV infection in patients with hematological malignancies undergoing chemotherapy and corticosteroid treatment. In our case, her liver function test was normal, image examination showed no signs of liver fibrosis, and liver biopsy revealed no significant hepatitis or liver fibrosis. However, close monitoring and management of coinfection with HCV are necessary and should be in coordination with therapy for MM.
In summary, we described a case of multiple space-occupying lesions in liver unexpectedly proved to be multiple myeloma. Liver lesions involving MM should be differentially diagnosed from other diseases.
| 1. | Thomas FB, Clausen KP, Greenberger NJ. Liver disease in multiple myeloma. Arch Intern Med. 1973;132:195-202. |
| 2. | Perez-Soler R, Esteban R, Allende E, Tornos Salomo C, Julia A, Guardia J. Liver involvement in multiple myeloma. Am J Hematol. 1985;20:25-29. |
| 3. | Talamo G, Cavallo F, Zangari M, Barlogie B, Lee CK, Pineda-Roman M, Kiwan E, Krishna S, Tricot G. Clinical and biological features of multiple myeloma involving the gastrointestinal system. Haematologica. 2006;91:964-967. |
| 4. | Oshima K, Kanda Y, Nannya Y, Kaneko M, Hamaki T, Suguro M, Yamamoto R, Chizuka A, Matsuyama T, Takezako N. Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol. 2001;67:1-5. |
| 5. | Thiruvengadam R, Penetrante RB, Goolsby HJ, Silk YN, Bernstein ZP. Multiple myeloma presenting as space-occupying lesions of the liver. Cancer. 1990;65:2784-2786. |
| 6. | Curtis JM, Pellegrini V, Tappin JA. Case report: multiple myeloma--a rare presentation. Clin Radiol. 1995;50:63-64. |
| 7. | Kelekis NL, Semelka RC, Warshauer DM, Sallah S. Nodular liver involvement in light chain multiple myeloma: appearance on US and MRI. Clin Imaging. 1997;21:207-209. |
| 8. | Fernandez-Flores A, Fortes J, Smucler A, Orduna M, Pol A. Involvement of the liver by multiple myeloma as nodular lesions: a case diagnosed by fine-needle aspiration and immunocytochemistry. Diagn Cytopathol. 2003;29:280-282. |
| 9. | Invernizzi R, Maffe GC, Travaglino E, Pagani E, Pieresca C. Nodular lesions of the liver in multiple myeloma. Haematologica. 2007;92:e81. |
| 10. | Walz-Mattmuller R, Horny HP, Ruck P, Kaiserling E. Incidence and pattern of liver involvement in haematological malignancies. Pathol Res Pract. 1998;194:781-789. |
| 11. | Kapadia SB. Multiple myeloma: a clinicopathologic study of 62 consecutively autopsied cases. Medicine (Baltimore). 1980;59:380-392. |
| 12. | Edwards GA, Zawadzki ZA. Extraosseous lesions in plasma cell myeloma. A report of six cases. Am J Med. 1967;43:194-205. |
| 13. | Lakatos PL, Fekete S, Horanyi M, Fischer S, Abonyi ME. Development of multiple myeloma in a patient with chronic hepatitis C: A case report and review of the literature. World J Gastroenterol. 2006;12:2297-2300. |
| 14. | Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K, Takahashi T, Oyama M. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158-165. |