Published online Oct 28, 2009. doi: 10.3748/wjg.15.5035
Revised: September 9, 2009
Accepted: September 15, 2009
Published online: October 28, 2009
AIM: To investigate the expression pattern of γ-synuclein in colorectal cancer (CRC) tissues, and to study the effects of γ-synuclein on CRC cell line HCT116 biological features in vitro.
METHODS: The expression pattern of γ-synuclein was determined in 54 CRC tissues and 30 tumor-matched nonneoplastic adjacent tissues (NNAT) 5 cm away from the tumor via real-time quantitative reverse transcription PCR (RT-PCR) and immunohistochemistry. The relationship between γ-synuclein protein expression and clinicopathological factors of CRC tissues was analyzed. Three small interfering RNA (siRNA) targeting γ-synuclein mRNA plasmids were constructed and transfected into the CRC cell line HCT116. The stable cell lines were selected with G-418 for 28 d, and the biological features of these cells were examined by cell growth curve, soft agar assay, and cell migration and invasion assays in vitro.
RESULTS: The expression of γ-synuclein mRNA and protein was much higher in CRC tissue samples than in NNAT samples (P = 0.02, P = 0.036). There was a significant correlation between the γ-synuclein protein expression and clinical stage and lymph node involvement of CRC (P = 0.02, P = 0.033). In functional analysis we found that down-regulation of γ-synuclein expression in HCT116 cells could inhibit the growth, colony formation rate, and migration and invasion ability of HCT116 cells.
CONCLUSION: Increased expression of γ-synuclein in CRC tissues and the biological effects of reduced γ-synuclein expression on HCT116 cells suggest that γ-synuclein may play a positive role in the progression of CRC.
- Citation: Ye Q, Feng B, Peng YF, Chen XH, Cai Q, Yu BQ, Li LH, Qiu MY, Liu BY, Zheng MH. Expression of γ-synuclein in colorectal cancer tissues and its role on colorectal cancer cell line HCT116. World J Gastroenterol 2009; 15(40): 5035-5043
- URL: https://www.wjgnet.com/1007-9327/full/v15/i40/5035.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5035
| Primer | Sequence (5’-3’) | Product size (bp) |
| γ-synuclein-5′ | GGAGGACTTGAGGCCATCTG | 73 (339 to 411) |
| γ-synuclein -3′ | CTCCTCTGCCACTTCCTCTTTC | |
| GAPDH-5′ | GGACCTGACCTGCCGTCTAG | 100 (831 to 930) |
| GAPDH-3′ | GTAGCCCAGGATGCCCTTGA |
| Oligonucleotides | Sequence (5’-3’) |
| siRNA1: Target sequence | AAGACCAAGGAGAATGTTGTA |
| Sense strand | 5'-GATCCGACCAAGGAGAATGTTGTATTCAAGAGATACAACATTCTCCTTGGTCTTTTTTGGAAA-3' |
| Antisense strand | 5'-AGCTTTTCCAAAAAAGACCAAGGAGAATGTTGTATCTCTTGAATACAACATTCTCCTTGGTCG-3' |
| siRNA2: Target sequence | AAGGAGAATGTTGTACAGAGC |
| Sense strand | 5'-GATCCGGAGAATGTTGTACAGAGCTTCAAGAGAGCTCTGTACAACATTCTCCTTTTTTGGAAA-3' |
| Antisense strand | 5'-AGCTTTTCCAAAAAAGGAGAATGTTGTACAGAGCTCTCTTGAAGCTCTGTACAACATTCTCCG-3' |
| siRNA3: Target sequence | AATGTTGTACAGAGCGTGACC |
| Sense strand | 5'-GATCCGTGTTGTACAGAGCGTGACCTTCAAGAGAGGTCACGCTCTGTACAACATTTTTTGGAAA-3' |
| Antisense strand | 5'-AGCTTTTCCAAAAAATGTTGTACAGAGCGTGACCTCTCTTGAAGGTCACGCTCTGTACAACACG-3' |
| Variable | n | γ-synuclein protein expression | P value1 | ||
| Positive | Negative | ||||
| Age | |||||
| ≥ 60 | 28 | 14 | 14 | ||
| < 60 | 26 | 12 | 14 | 0.793 | |
| Sex | |||||
| Male | 32 | 14 | 18 | ||
| Female | 22 | 12 | 10 | 0.580 | |
| Histological types | |||||
| Differentiated | 47 | 21 | 26 | ||
| Undifferentiated | 7 | 5 | 2 | 0.243 | |
| Stage | |||||
| I | 13 | 2 | 11 | ||
| II | 11 | 5 | 6 | ||
| III | 24 | 14 | 10 | ||
| IV | 6 | 5 | 1 | 0.020 | |
| Lymph node invision | |||||
| Positive | 29 | 18 | 11 | ||
| Negative | 25 | 8 | 17 | 0.033 | |
| Distant metastasis | |||||
| Positive | 6 | 5 | 1 | ||
| Negative | 48 | 21 | 27 | 0.095 | |
The synucleins are a family of small, soluble, highly conserved neuronal proteins that consist of α-, β-, and γ-synuclein. They are a natively unfolded group of proteins that are characterized by 5-6 repeats of the amino acid motif (KTKEGV), constituting most of the N-terminal half of the proteins[1-3]. The synucleins have attracted considerable attention due to their involvement in neurodegenerative diseases. α-synuclein is the major component of Lewy bodies in Parkinson’s disease and has also been identified as the non-amyloid component of amyloid deposition, the hallmark of Alzheimer’s disease[4,5]. β- and γ-synuclein are assumed to have a neuroprotective role by inhibiting α-synuclein aggregation and toxicity[6,7].
γ-synuclein gene [also referred to as breast carcinoma specific gene 1 (BCSG1)] initially was cloned from infiltrating breast carcinoma cells by using the expressed sequence tag-based differential cDNA sequencing approach[8]. γ-synuclein maps to chromosome region 10q23, and is composed of five exons and transcribed into an mRNA of about 1 kb, coding 127 amino acids[9]. γ-synuclein expression is usually highly tissue-specific and restricted to brain tissue and presynaptic terminals[2]. However, the tissue-specificity appears to be lost, and γ-synuclein is abnormally expressed in a high percentage of advanced breast and ovarian cancers, but not in normal or benign tissues[10]. Furthermore, overexpression of γ-synuclein can stimulate proliferation, and induce invasion and metastasis of breast cancer cells[11]. γ-synuclein has also been shown to compromise normal mitotic checkpoint controls, resulting in multinucleation as well as faster breast cancer cell growth[12,13]. Liu et al[14] found that γ-synuclein protein was also abnormally expressed in a high percentage of tumor tissues of other cancer types, including liver, gastric, lung, prostate, cervical etc., but rarely expressed in tumor-matched nonneoplastic adjacent tissues (NNAT). However, Zhou et al[15] had an opposite conclusion in esophagus cancer, in which low expression levels of γ-synuclein in human esophageal squamous cell carcinoma (ESCC) and biological effects of γ-synuclein overexpression on ESCC 9706 cells suggested that γ-synuclein might play a role as a negative regulator in the development of human ESCC. Therefore, further study in cancer tissues and cell line culture is needed to understand the roles of γ-synuclein in the development of other human neoplastic diseases.
Recent reports demonstrate that colorectal cancer (CRC) has been the third most common malignancy and the third leading cause of cancer-related deaths worldwide[16]. The conventional therapies involving surgery and adjuvant therapy seem to give rise to improvements in progression-free and overall survival; nevertheless about 50% of patients die within 5 years owing to metastasis or recurrent disease. Patients with early stage CRC have an estimated 5 year survival rate of 91%, compared to only 6% for those with later stage disease. Early detection remains the most important factor in improving long-term survival. Furthermore, tumor invasion and regional lymph node metastasis are important factors for determining CRC prognosis[17-19].
To further determine whether aberrant expression of γ-synuclein is involved in the development of CRC, and identify a new biomarker or a potential target for diagnosis and treatment, we examined expression patterns of γ-synuclein in CRC tissues, analyzed the relationship between γ-synuclein protein expression and clinicopathological factors of CRC, and then studied the effects of γ-synuclein down-regulation on colorectal cancer cell line HCT116 biological features in vitro.
Fifty-four CRC samples and 30 NNAT samples 5 cm away from the tumor were obtained from patients undergoing CRC surgery between January 2005 and October 2008 at the Department of General Surgery, Ruijin Hospital, Shanghai, China. After washing with RNase-free 9 mL/L NaCl to remove blood after surgery, one half of each sample was snap-frozen in liquid nitrogen immediately and stored at -80°C for RNA extraction, and the other half was fixed in 40 g/L formalin for histological assessment. For tumor samples, non-tumor portions were trimmed off from the frozen tumor blocks and the selected areas had more than 80% tumor cells as shown by histological assessment. Tumors were staged using the TNM and World Health Organization classification systems. The ethics committee at Ruijin Hospital approved the use of these tissues for research purposes. The colorectal cancer cell line HCT116 (ATCC No. CCL-247) was grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Life Technologies Inc, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Summit Biotechnology, Fort Collins, CO, USA). The HCT116 cells were maintained in a humidified incubator at 37°C with 50 mL/L CO2, fed every 3 d with complete medium, and subcultured when confluence was reached.
Cultured cells were washed twice with phosphate-buffered saline (PBS) and harvested, and tissues were ground into fine powder in liquid nitrogen before extraction of RNA. Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis from 1 μg of RNA was performed with a reverse transcription system kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Real-time quantitative PCR was carried out in 96-well polypropylene microplates on an ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA) using SYBR Green Realtime PCR Master Mix (TOYOBO, Tokyo, Japan) according to the manufacturer’s instructions. Amplification was carried out with the following profile: 1 cycle at 95°C for 1 min, and 40 cycles each at 95°C for 15 s, 59°C for 15 s, 72°C for 45 s. All PCR reactions were performed in triplicate wells. Specificity of the resulting PCR products was confirmed by melting curves. H2O was used as a negative control. Data were analyzed by using the comparative Ct (ΔCt) method and the amount of γ-synuclein relative to GAPDH was expressed as 10 000 × 2-(ΔCt). Table 1 provides the sequences of the primers used in these studies.
Unstained 4 mm sections were cut from the tissue paraffin-embedded block and deparaffinized in xylene, and the slides were bathed in 0.01 mol/L sodium citrate and heated in a microwave oven for 12 min. The sections were incubated with mouse anti-γ-synuclein monoclonal antibody (SantaCruz, CA, USA) at a dilution of 1:100 and kept at 4°C overnight. Negative control slides were treated with non-immunized mouse immunoglobulin fraction under equivalent conditions. For the secondary developing reagents, a labeled streptavidin-biotin kit (DAKO, CA, USA) was used. Slides were developed with diaminobenzaminidine and counterstained with hematoxylin. Positive cases were defined by the presence of intracellular staining with red/brown color in epithelial cells. Negative cases were defined by the absence of specific intracellular staining as seen in negative controls. Samples were evaluated under light microscopy independently by two pathologists without prior knowledge of the patients’ clinical data.
The pGCsi-U6/neo/GFP plasmid (Shanghai GeneChem., Ltd, Shanghai, China), a siRNA expression vector containing a green fluorescent protein gene (GFP) under a separate promoter for tracking the transfection efficiency, was used for the cloning of small synthetic oligonucleotides that encode two complementary sequences of 19 nucleotides separated by a short spacer region of 9 nucleotides. Three sequences (as shown in Table 2) unique to the coding region of γ-synuclein were designed and inserted between the BamHI and HindIII sites of the pGCsi-U6/neo/GFP plasmid. The positive clones were confirmed by sequencing.
One day before transfection, HCT116 cells were plated in a six-well plate with 1 × 105 cells per well using culture medium without antibiotics. The cells were transfected with 3.0 μg/well of pGCsi-U6/neo/GFP-vector and pGCsi-siRNA plasmids, respectively, using Lipofectamine (Invitrogen) according to the manufacturer’s protocol. Cells transfected with medium but lacking DNA served as controls. Fresh growth medium was replaced after 4 h of transfection. Cells were passaged at a 1:10 dilution at 24 h after transfection and cultured in medium supplemented with G418 (Promega, Madison, WI) at 1000 μg/mL for 4 wk. Stably transfected clones were picked and maintained in medium containing 400 μg/mL G418 for further study.
Cells were harvested and lysed with mammalian protein extraction reagent (Pierce Rockford, IL, USA). Protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (Pierce Rockford, IL, USA). Samples containing 50 μg of protein were mixed with 2 × sodium dodecyl sulfate (SDS) gel-loading buffer (100 mmol/L Tris-CL, 200 mmol/L dithiothreitol, 4% SDS, 0.2% bromophenol blue, and 20% glycerol), boiled for 5 min, loaded onto each lane of 15% acrylamide gel in a minigel apparatus (Bio-Rad, Richmond, CA, USA), and separated by SDS-PAGE. The separated proteins were electrophoretically transferred to a Sequi-blot PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). After being incubated with mouse anti-γ-synuclein monoclonal antibody (SantaCruz, CA, USA) (1:500), and goat anti-mouse IgG-AP antibody (SantaCruz, CA, USA) (1:5000) respectively, immune complexes were detected using BCIP/NBT Alkaline Phosphatase Color Development Kit (Sigma, St. Louis, MO). GAPDH served as a loading control.
Cells were seeded onto 96-well plates at a density of 2 × 103 cells per well in 100 μL medium containing 10% FBS. The number of viable cells was determined daily with WST-8 cytotoxicity assay using the Cell Counting Kit-8 (Dojindo, Japan). Briefly, 10 μL of the CCK-8 solution was added to each well of the microplate, and the absorbance at 490 nm was measured by a microplate reader (μQuant, Bio-Tek, USA) after 4 h incubation.
Cells (1 × 103) were trypsinized to a single-cell suspension and then plated in triplicate onto six-well plates in complete culture medium containing 0.3% agar on top of 0.6% agar in the same medium. Cultures were maintained at 37°C in the 50 mL/L CO2 incubator for 15 d. The colonies were fixed with 70% ethanol, and stained with 0.2% crystal violet. The colonies containing at least 50 cells were counted. Colony formation rates were calculated as the number of colonies relative to that of cells initially plated in a well (1 × 103), and expressed as mean ± SD.
Boyden chambers with 8 μm polycarbonate membranes in 24-well dishes (Nucleopore, Pleasanton, CA) were used for migration assay, and chambers coated with 4 mg/mL growth factor reduced Matrigel (50 μg; Collaborative Biomedical, Becton Dickinson Labware) were used for the invasion assay. Cells (1 × 105) were resuspended in serum-free DMEM and added to the upper chamber in triplicate. Consecutively, DMEM with 10% FBS was added to the lower chamber. Chambers were incubated at 37°C in the 50 mL/L CO2 incubator for 24 h. After incubation, the chambers were fixed with 70% ethanol, and stained with 0.2% crystal violet. Cells on the surface of the upper chamber were removed by swiping with cotton swabs. The amount of migration and invasion cells in the lower chamber was determined under light microscopy. The data are means ± SD of counting ten random fields of vision.
Statistical analyses were performed using SPSS11.0 software (Shanghai jiaotong University School of Medicine, Shanghai, China). Mann-Whitney U-test was used to analyze γ-synuclein mRNA expression in paired CRC and NNAT samples. The Fisher’s exact test was used to test the significance of the difference in frequency of γ-synuclein protein expression between CRC and NNAT samples, and to assess the relationship between the protein expression and clinicopathological characteristics of CRC. Two-way analysis of variance (ANOVA) was performed to detect the effects of γ-synuclein knockdown on cell proliferation, soft agar colony formation, cell migration and invasion, and Student-Newman-Keuls test was used to detect the difference between any two groups. P < 0.05 was selected as the statistically significant value.
γ-synuclein mRNA expression in 54 CRC and 30 NNAT samples was examined using Q-RT-PCR. Table 3 shows the results of Q-RT-PCR. γ-synuclein mRNA expression in CRC samples ranged from 1.12 to 39.36 with a median value of 11.06, while in matched NNAT samples it ranged from 0.81 to 30.21 with a median value of 6.11. The γ-synuclein mRNA expression levels in CRC samples were significantly higher than those in NNAT samples (P = 0.02).
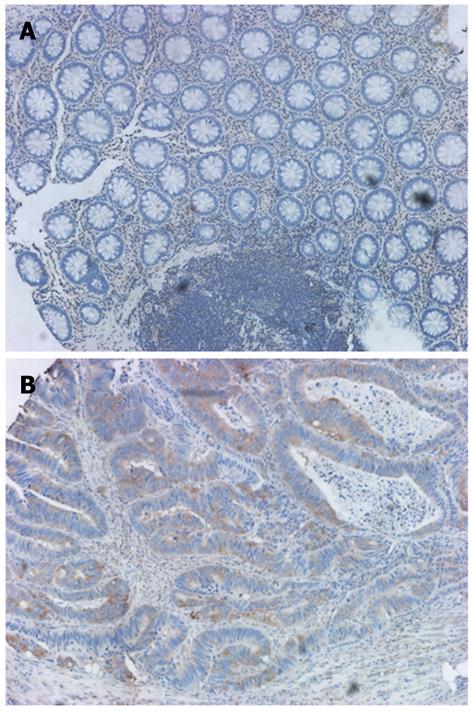
The expression and subcellular localization of γ-synuclein protein were evaluated via IHC in 54 CRC and 30 NNAT samples. NNAT sections showed either no protein expression (n = 23) or relatively weak protein expression (n = 7) in the cytoplasm of epithelium cells (Figure 1A). Conversely, the immunoreactive patterns of γ-synuclein were predominantly positively identified in the cytoplasm, sometimes in the nucleus of cancer cells (Figure 1B) with a relatively high frequency of 48.1% (Table 3, P = 0.036).
In the analysis of γ-synuclein protein expression in CRC tissues and various CRC patients’ clinicopathologic variables, the results clearly showed a close association of γ-synuclein staining with clinical stage and lymph node involvement (Table 4). The frequency of positive γ-synuclein staining was much higher in later stage tumors than in earlier stage tumors (P = 0.02), and was much higher in lymph node-positive tumors than in lymph node-negative ones (P = 0.033). However, there was no significant correlation between the γ-synuclein protein expression and other clinicopathologic characteristics.
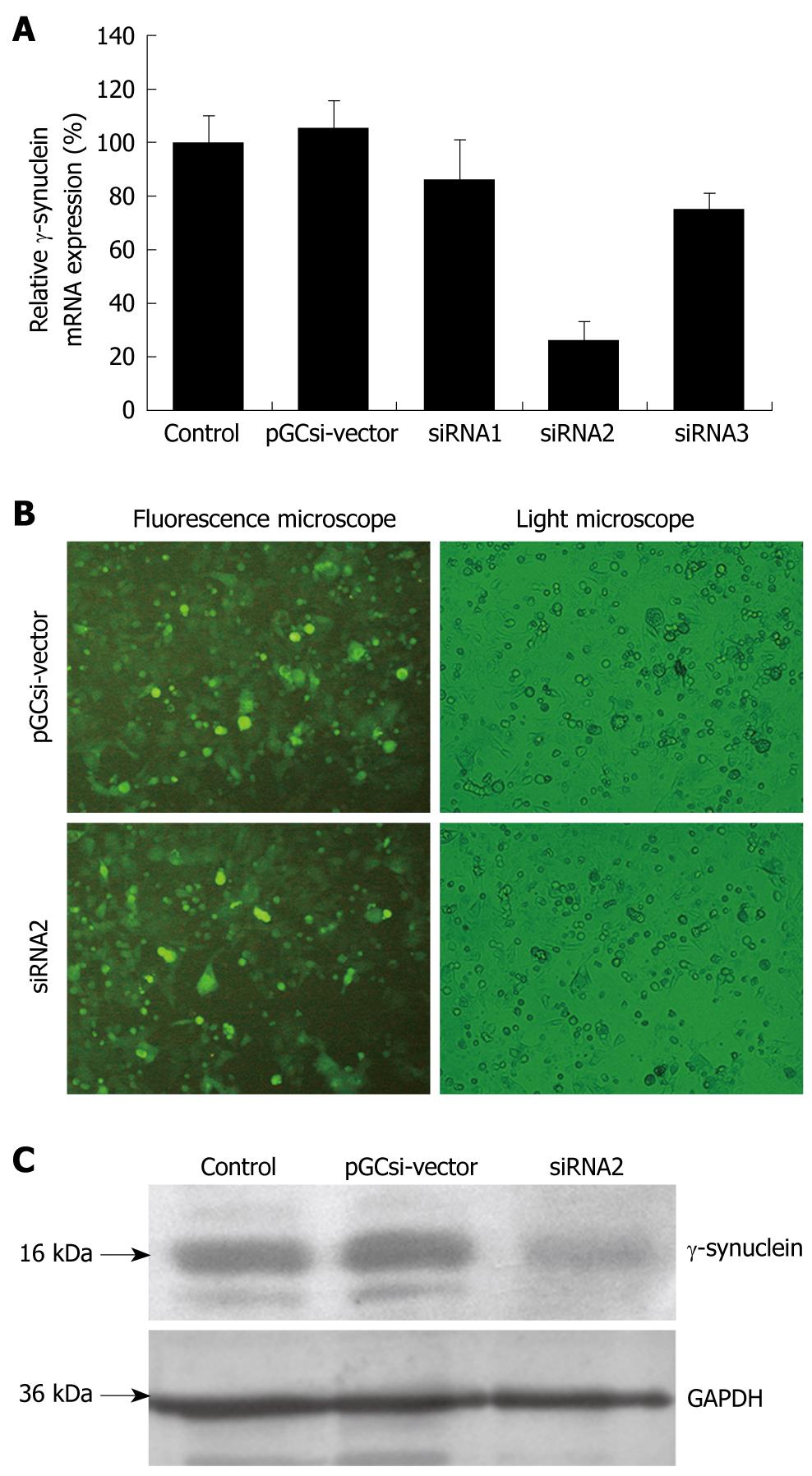
We first investigated three recombinant γ-synuclein-specific siRNA plasmids, pGCsi-siRNA1, pGCsi-siRNA2, and pGCsi-siRNA3. These γ-synuclein-specific siRNA plasmids and pGCsi-U6/neo/GFP-vectors were transfected into HCT116 cells. After 24 h, these cells were examined for γ-synuclein expression by Q-RT-PCR. As shown in Figure 2A, γ-synuclein levels were different in the transfected HCT116 cells containing siRNA1, siRNA2, siRNA3 and the vector. There were no significant changes of γ-synuclein mRNA expression in pooled HCT116/vector, HCT116/siRNA1, and HCT116/RNA3 cells. However, in HCT116/siRNA2 cells, γ-synuclein mRNA levels were significantly low, compared with parental HCT116 cells and HCT116/vector cells. Then stable transfected clones of HCT116/siRNA2 cells were selected with G418. After 4 wk of the selection, stable transfected clones were established (Figure 2B). These clones were examined for γ-synuclein expression by western blotting, the result of which suggested that pGCsi-siRNA2 plasmid could specifically knock down γ-synuclein protein expression in the stable transfected HCT116 cells (Figure 2C).
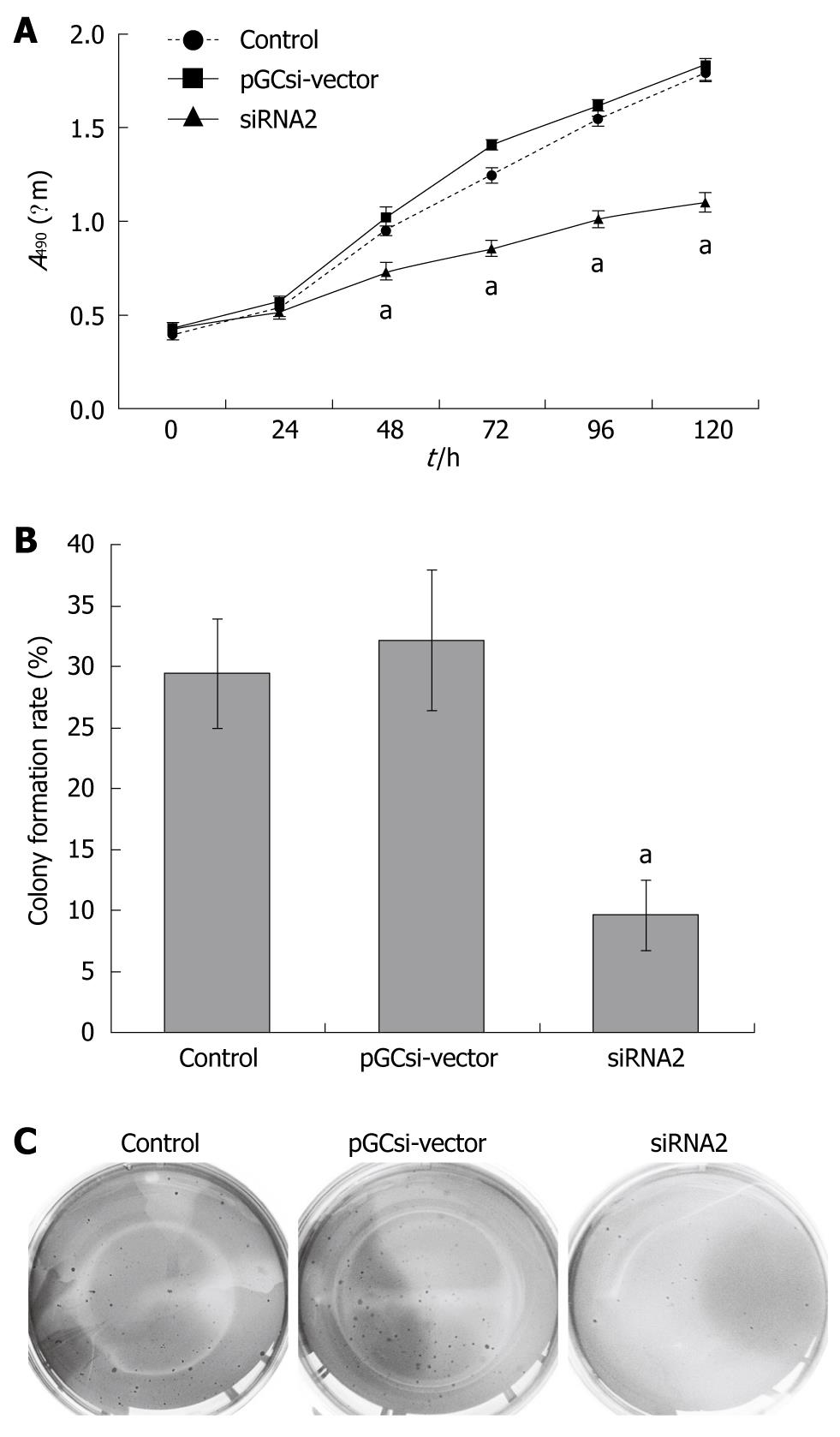
In vitro cell proliferation tests, two clones derived from stable transfectants with control vector, siRNA2 plasmids, and parental HCT116 cells were chosen for further study. As shown in Figure 3A, γ-synuclein knockdown suppressed cancer cell growth significantly in regular medium. The number of pooled HCT116/siRNA2 cells was significantly reduced by 48, 72, 96, and 120 h after plating, respectively, compared with the HCT116/vector and parental HCT116 cells (P < 0.05).
Subsequent soft agar colony formation assay was done to evaluate the tumorigenicity of γ-synuclein down-regulated cells in vitro. Colony formation rates were 29.4% ± 4.5%, 32.1% ± 5.8% and 9.6% ± 2.9% in parental HCT116 cells, HCT116/ vector and HCT116/siRNA2 cells (Figure 3B, P < 0.05). The size of colonies formed by HCT116/siRNA2 cells was much smaller than that of two control cells, and there were no significant differences between parental HCT116 cells and HCT116/vector cells colonies both in number and size (Figure 3C).
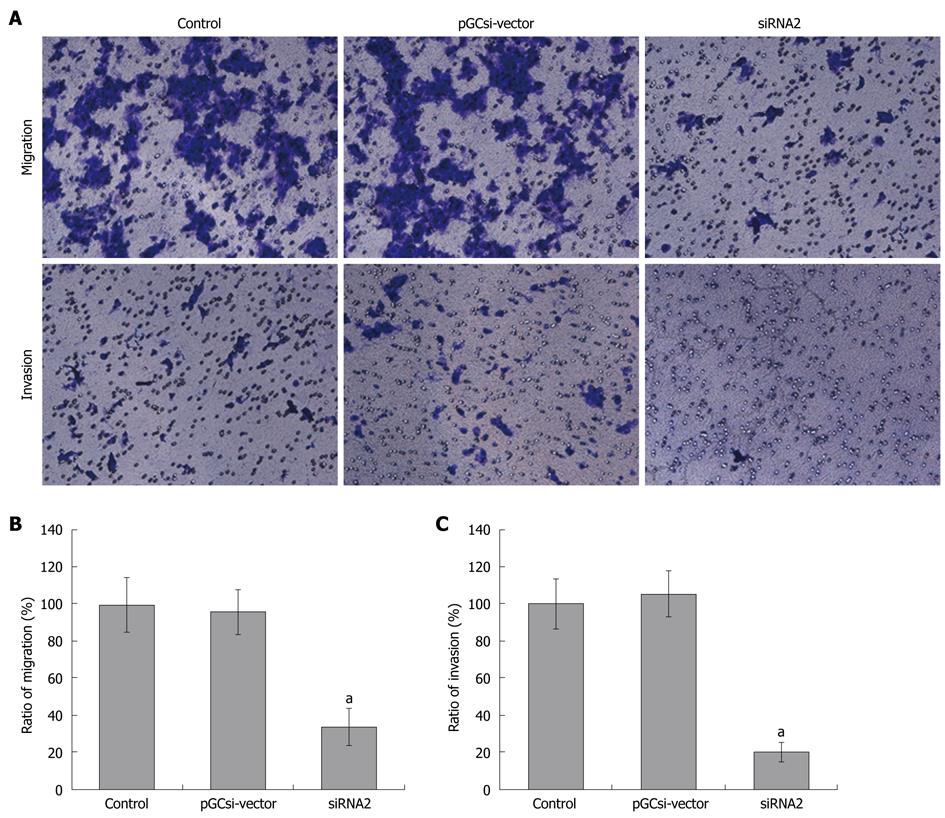
The close correlation of γ-synuclein protein expression and CRC staging suggests that γ-synuclein might be involved in advanced stage tumor progression and metastasis. We used an in vitro reconstituted basement membrane (Matrigel) invasion assay to determine the effect of γ-synuclein on cell migration and invasion (Figure 4A). The results showed that the amount of migration HCT116/siRNA2 cells in the lower chamber was much less than that of parental HCT116 cells, and HCT116/vector cells (Figure 4B, P < 0.05). It was demonstrated that γ-synuclein down-expression led to suppression of cell motility in HCT116 cells. Similarly, we observed that γ-synuclein down-expression led to decreased cell invasion in HCT116 cells. HCT116/siRNA2 cells showed a significant decrease in the number of invasive cells compared to that of two control cells (Figure 4C, P < 0.05).
γ-synuclein belongs to the synuclein protein family, consisting of α-, β-, and γ-synuclein, which are abundantly expressed in nervous tissues[9]. Although there has been a report that showed down-regulation of γ-synuclein in human ESCC, more studies support the statement of over-expression of γ-synuclein in more types of cancer[14,15,20-22]. The loss of tissue-specificity raises questions about the involvement of γ-synuclein in the process of tumorigenesis and metastasis, and presents the possibility to use γ-synuclein as a potential target for early diagnosis and treatment. However, little is known about the expression and biological effects of γ-synuclein in CRC.
In the current study, we showed that γ-synuclein expression levels were higher in CRC tissues than those in matched NNAT. IHC analysis also confirmed that CRC tissues exhibited abundant γ-synuclein expression in the cytoplasm of cancer cells, in contrast to NNAT, which did not appear to exhibit γ-synuclein expression or exhibit faint expression.
To further investigate how γ-synuclein contributes to the biological behavior changes in CRC, we constructed specific γ-synuclein siRNA plasmids and established permanent transfected HCT116 cells to investigate the potential role of γ-synuclein in the progression of CRC. Consistent with our observation that the γ-synuclein expression levels were lower in NNAT than in CRC tissues, the cell growth and colony formation rate decreased in HCT116/siRNA2 cells with reduced expression of γ-synuclein, compared with parental HCT116 cells, and HCT116/vector cells, which gave evidence that γ-synuclein indeed had the ability to promote cell growth. Previous studies have demonstrated that ectopic expression of γ-synuclein increased breast cancer cell growth in anchorage dependent and independent conditions through interaction with BubR1, a mitotic checkpoint kinase, which led to inhibition of the mitotic checkpoint control[12,13,23]. It has also been shown that γ-synuclein could constitutively activate ERK1/2, and increase ER-α transcriptional activity through an HSP-based multiprotein chaperone complex, which led to an increase in breast cancer or ovarian cell survival and proliferation[24-28].
In addition to the effects on cell growth, γ-synuclein is associated with cell invasion and metastasis. In previous in vitro studies, retinoblastoma cell lines overexpressing γ-synuclein were shown to have higher MMP9 protein levels and activity, which were enhanced in cell motility and invasion[29]. In in vivo studies, γ-synuclein was also shown to cause metastasis in nude mice on implanting γ-synuclein expressing MDA-MB 435 breast cancer cells in fat pads of these mice. IHC results showed mice given implants of γ-synuclein positive cells displayed an increase in tumor growth, and metastasis into axillary lymph nodes and lungs, compared with mice given control implants[11]. In the current study, we presented the clinical evidence and experimental data to indicate that γ-synuclein played a key role in CRC invasion and metastasis. We analyzed the relationship between γ-synuclein protein expression in 54 CRC tissues and the clinicopathologic characteristics of patients with CRC, and found that the frequency of positive γ-synuclein staining was much higher in tumors with lymph node-positive or later stage than in lymph node-negative or earlier stage tumors (P < 0.05). Our results also showed that there was a tendency for high γ-synuclein expression with metastasis. However, this difference is not significant (P = 0.095), possibly because of our relatively small sample size. Consistent with the clinical evidence, we also observed that reduced γ-synuclein expression led to decreased cell motility and invasion in HCT116 cells. All these results gave evidence that γ-synuclein may indeed function as key mediators of cancer cell growth and metastasis and will be a promising target for CRC treatment. Biological treatment targeting γ-synuclein has been studied in breast cancer, and Singh et al[30] have designed and characterized a γ-synuclein targeting peptide inhibitor, which associates with γ-synuclein and enhances sensitivity of breast cancer cells to antimicrotubule drugs.
In summary, we have shown that strong expression of γ-synuclein occurred in CRC tissues and correlated with the advancement of tumor stage and lymph node involvement. With a vector-based siRNA method, we showed that stable down-regulation of γ-synuclein expression inhibited CRC cell growth, colony formation, motility and invasion. Therefore, γ-synuclein is likely to play an important role in the progression of CRC. Further study is needed to prove the value of γ-synuclein as a biomarker or molecular target for CRC diagnosis, prognosis evaluation and therapy. The following research may encompass: 1. Examination of γ-synuclein expression in serum or stool samples from patients with CRC; 2. Relationship between γ-synuclein expression and 5-year survival rate of CRC patient; 3. Delineation of the interaction between γ-synuclein and other proteins, and γ-synuclein targeting biotherapy in cell culture and animal model.
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related deaths worldwide. The conventional therapies involving surgery and adjuvant therapy do not significantly prolong the survival period. It is necessary to identify a reliable biomarker or a potential target for diagnosis and treatment.
γ-synuclein, a member of synuclein protein family, is abundantly expressed in brain tissue and presynaptic terminals. However, the tissue specificity of γ-synuclein expression appears to be lost in some types of cancer. Particularly in breast cancers, γ-synuclein promotes malignancy of breast cancer cell lines in in vitro studies and animal models. However, little is known about γ-synuclein in colorectal cancer.
The results of this study provide strong evidence suggesting that γ-synuclein expression is up-regulated in CRC tissues, and is significantly correlated with clinical stage and lymph node involvement of CRC. The authors also constructed specific γ-synuclein siRNA plasmids and established a permanent transfected colorectal cancer cell line HCT116, and found that down-regulation of expression of γ-synuclein in HCT116 cells could inhibit the growth, colony formation rate, and migration and invasion ability of HCT116 cells.
These results demonstrate that γ-synuclein indeed may function as a key mediator of cancer cell growth and metastasis and will be a promising target for CRC diagnosis, prognosis evaluation and biotherapy.
Mitotic checkpoint is a cellular inherent mechanism, which strictly controls the cell division cycle and makes sure there is faithful cell replication. G418, a kind of aminoglycoside antibiotic, is a most common resistance selection reagent, used for stable transfectants in molecular biology tests.
The paper investigated expression pattern of γ-synuclein in CRC tissues, and the effects of γ-synuclein on CRC cell line HCT116 biological features also were studied in vitro. The study is well conducted and the results is clear.
We are grateful to Dr. Feng Zhang (Institute of Digestive Surgery, Ruijin Hospital, China) for excellent technical assistance and advice.
| 1. | Lavedan C, Leroy E, Dehejia A, Buchholtz S, Dutra A, Nussbaum RL, Polymeropoulos MH. Identification, localization and characterization of the human gamma-synuclein gene. Hum Genet. 1998;103:106-112. |
| 2. | Lavedan C. The synuclein family. Genome Res. 1998;8:871-880. |
| 3. | Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249-254. |
| 4. | Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282-11286. |
| 5. | Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839-840. |
| 6. | Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277:11970-11978. |
| 7. | Park JY, Lansbury PT Jr. Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson's disease. Biochemistry. 2003;42:3696-3700. |
| 8. | Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, Joseph BK, Rosen C, Shi YE. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997;57:759-764. |
| 9. | Ahmad M, Attoub S, Singh MN, Martin FL, El-Agnaf OM. Gamma-synuclein and the progression of cancer. FASEB J. 2007;21:3419-3430. |
| 10. | Wu K, Weng Z, Tao Q, Lin G, Wu X, Qian H, Zhang Y, Ding X, Jiang Y, Shi YE. Stage-specific expression of breast cancer-specific gene gamma-synuclein. Cancer Epidemiol Biomarkers Prev. 2003;12:920-925. |
| 11. | Jia T, Liu YE, Liu J, Shi YE. Stimulation of breast cancer invasion and metastasis by synuclein gamma. Cancer Res. 1999;59:742-747. |
| 12. | Gupta A, Inaba S, Wong OK, Fang G, Liu J. Breast cancer-specific gene 1 interacts with the mitotic checkpoint kinase BubR1. Oncogene. 2003;22:7593-7599. |
| 13. | Inaba S, Li C, Shi YE, Song DQ, Jiang JD, Liu J. Synuclein gamma inhibits the mitotic checkpoint function and promotes chromosomal instability of breast cancer cells. Breast Cancer Res Treat. 2005;94:25-35. |
| 14. | Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C, Wang L, Zhao W, Jiang JD, Liu J. Loss of epigenetic control of synuclein-gamma gene as a molecular indicator of metastasis in a wide range of human cancers. Cancer Res. 2005;65:7635-7643. |
| 15. | Zhou CQ, Liu S, Xue LY, Wang YH, Zhu HX, Lu N, Xu NZ. Down-regulation of gamma-synuclein in human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:1900-1903. |
| 16. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. |
| 17. | Gennari L, Russo A, Rossetti C. Colorectal cancer: what has changed in diagnosis and treatment over the last 50 years? Tumori. 2007;93:235-241. |
| 18. | Samoha S, Arber N. Cyclooxygenase-2 inhibition prevents colorectal cancer: from the bench to the bed side. Oncology. 2005;69 Suppl 1:33-37. |
| 19. | McGartland LP, Mulcahy MF, Benson AB 3rd. Pre- and postoperative adjuvant therapy for locally advanced rectal cancer. Clin Adv Hematol Oncol. 2004;2:806-814. |
| 20. | Li Z, Sclabas GM, Peng B, Hess KR, Abbruzzese JL, Evans DB, Chiao PJ. Overexpression of synuclein-gamma in pancreatic adenocarcinoma. Cancer. 2004;101:58-65. |
| 21. | Zhao W, Liu H, Liu W, Wu Y, Chen W, Jiang B, Zhou Y, Xue R, Luo C, Wang L. Abnormal activation of the synuclein-gamma gene in hepatocellular carcinomas by epigenetic alteration. Int J Oncol. 2006;28:1081-1088. |
| 22. | Fung KM, Rorke LB, Giasson B, Lee VM, Trojanowski JQ. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106:167-175. |
| 23. | Mao Y, Abrieu A, Cleveland DW. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell. 2003;114:87-98. |
| 24. | Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J Biol Chem. 2002;277:35050-35060. |
| 25. | Pan ZZ, Bruening W, Godwin AK. Involvement of RHO GTPases and ERK in synuclein-gamma enhanced cancer cell motility. Int J Oncol. 2006;29:1201-1205. |
| 26. | Jiang Y, Liu YE, Lu A, Gupta A, Goldberg ID, Liu J, Shi YE. Stimulation of estrogen receptor signaling by gamma synuclein. Cancer Res. 2003;63:3899-3903. |
| 27. | Liu YE, Pu W, Jiang Y, Shi D, Dackour R, Shi YE. Chaperon-ing of estrogen receptor and induction of mammary gland proliferation by neuronal protein synuclein gamma. Oncogene. 2007;26:2115-2125. |
| 28. | Jiang Y, Liu YE, Goldberg ID, Shi YE. Gamma synuclein, a novel heat-shock protein-associated chaperone, stimulates ligand-dependent estrogen receptor alpha signaling and mammary tumorigenesis. Cancer Res. 2004;64:4539-4546. |
| 29. | Surgucheva IG, Sivak JM, Fini ME, Palazzo RE, Surguchov AP. Effect of gamma-synuclein overexpression on matrix metalloproteinases in retinoblastoma Y79 cells. Arch Biochem Biophys. 2003;410:167-176. |
| 30. | Singh VK, Zhou Y, Marsh JA, Uversky VN, Forman-Kay JD, Liu J, Jia Z. Synuclein-gamma targeting peptide inhibitor that enhances sensitivity of breast cancer cells to antimicrotubule drugs. Cancer Res. 2007;67:626-633. |
Peer reviewer: Vincent W Yang, Professor and Director, 201 Whitehead Research Building, 615 Michael Street, Atlanta, GA 30322, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Lin YP