Published online Oct 28, 2009. doi: 10.3748/wjg.15.5044
Revised: September 12, 2009
Accepted: September 19, 2009
Published online: October 28, 2009
AIM: To investigate the effects of class I phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 on the invasiveness and related mechanisms of implanted tumors of SGC7901 human gastric carcinoma cells in nude mice.
METHODS: Nude mice were randomly divided into model control groups and LY294002 treatment groups. On days 5, 10 and 15 after treatment, the inhibitory rate of tumor growth, pathological changes in tumor specimens, expression levels of matrix metalloproteinase (MMP)-2, MMP-9, CD34 [representing microvessel density (MVD)] and vascular endothelial growth factor (VEGF), as well as apoptosis indexes in tumor samples were observed.
RESULTS: In this study, we showed that treating the tumors with LY294002 could significantly inhibit carcinoma growth by 11.3%, 29.4% and 36.7%, after 5, 10 and 15 d, respectively, compared to the control group. Hematoxylin & eosin staining indicated that the rate of inhibition increased progressively (23.51% ± 3.11%, 43.20% ± 3.27% and 63.28% ± 2.10% at 5, 10 and 15 d, respectively) along with apoptosis. The expression of MMP-2 was also downregulated (from 71.4% ± 1.6% to 47.9% ± 0.7%, 31.9% ± 0.9% and 7.9% ± 0.7%). The same effects were observed in MMP-9 protein expression (from 49.4% ± 1.5% to 36.9% ± 0.4%, 23.5% ± 0.9% and 7.7% ± 0.6%), the mean MVD (from 51.2% ± 3.1% to 41.9% ± 1.5%, 30.9% ± 1.7% and 14.9% ± 0.8%), and the expression of VEGF (from 47.2% ± 3.1% to 25.9% ± 0.5%, 18.6% ± 1.2% and 5.1% ± 0.9%) by immunohistochemical staining.
CONCLUSION: The class I PI3K inhibitor LY294002 could inhibit the invasiveness of gastric cancer cells by downregulating the expression of MMP-2, MMP-9, and VEGF, and reducing MVD.
-
Citation: Xing CG, Zhu BS, Fan XQ, Liu HH, Hou X, Zhao K, Qin ZH. Effects of LY294002 on the invasiveness of human gastric cancer
in vivo in nude mice. World J Gastroenterol 2009; 15(40): 5044-5052 - URL: https://www.wjgnet.com/1007-9327/full/v15/i40/5044.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5044
Gastric cancer is the fourth most frequently diagnosed malignancy worldwide, accounting for 12% of all cancer-related deaths. In Asia and parts of South America in particular, gastric cancer is the most common epithelial malignancy and a leading cause of cancer-related death[1,2]. The major cause of death from gastric cancer is metastasis that is usually resistant to conventional treatment.
Invasiveness and metastasis are the leading biological characteristics of a malignant tumor, and have a close relation with factors such as movement of tumor cells, apoptosis and metastasis-associated genes. Matrix metalloproteinase (MMP)-2, MMP-9, intratumoral microvessel density (MVD) and vascular endothelial growth factor (VEGF) are important angiogenic factors, which have a higher expression in tumor tissues, may induce angiogenesis in the tumor and play an important role in metastases, invasion and prognosis of gastric cancer[3-7]. However, the underlying mechanism remains uncertain.
The phosphatidylinositol 3-kinase (PI3K) pathway plays a central role in the regulation of cell proliferation, growth, differentiation and survival[8,9]. Dysregulation of this pathway is frequently observed in a variety of tumors, including brain tumors and breast, ovarian and other carcinomas[10-12]. Therefore, inhibition of PI3K signaling is under investigation as a potentially useful approach for cancer treatment. However, the detailed mechanisms are poorly understood.
LY294002 is a specific inhibitor of class I PI3K. The antitumor activity of LY294002 may be related to the induction of apoptosis of tumor cells, but the precise mechanism of its antitumor activity is not well understood.
In the present study, we investigated whether the inhibition of class I PI3K by LY294002 restricted the growth and invasiveness of implanted tumors of SG7901 cells in nude mice. Through this research, we would suggest that the class I PI3K inhibitor LY294002 could play an important role in the inhibition of progression of gastric carcinoma, and may be of value in effective clinical antitumor therapy.
SG7901 cells and female Balb/c nude mice (4 wk old, 16-18 g) were purchased from the Chinese Academy of Sciences (Shanghai, China). RPMI-1640 medium was purchased from Gibco (Rockville, MD, USA). Fetal bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Material Co. (Hangzhou, China), anti-MMP-2 (sc-13595), anti-MMP-9 (sc-21733), anti-CD34 (sc-19621) and anti-VEGF (sc-57496) monoclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (USA). SP kit was purchased from Maixin Biotechnology (Fuzhou, China).
LY2940029 was purchased from Cell Signaling Technology (Beverly, MA, USA) and diluted in phosphate buffered saline (PBS) to create a stock solution that was stored according to the manufacturer’s instructions. The final concentration of the LY294002 solution used was 500 μmol/L. This concentration of LY294002 was selected on the basis of our experiments on implanted tumors of human SGC7901 cells in nude mice.
SGC7901 cells were maintained in RPMI-1640 medium (Gibco) containing 10% heat-inactivated fetal bovine serum (Hangzhou Sijiqing Biological Engineering Material Co.), 0.03% L-glutamine (Sigma) and incubated in a 5% CO2 atmosphere at 37°C. Cells in the mid-log phase were used in the experiments.
A transplanted tumor model was established by injecting 1 × 109 cells/mL human SGC7901 into the subcutaneous tissue in the armpit of nude mice. After 10 d, 25 nude mice were randomly divided into four groups, and 0.2 mL normal saline solution, LY294002 (500 μmol/L) was directly injected adjacent to the tumor, twice at a 2 d interval (5 d group), three times at 2 d intervals (10 and 15 d group). There were six nude mice given saline, 19 nude mice given LY294002 (six in the 5 d group, six in the 10 d group, seven in the 15 d group). Changes in tumor volume = (π/6) × abc (a: the length of the tumor, b: the width of the tumor and c: the depth of the tumor) were measured at 5, 10, 15 d after LY249002 treatment and the tumor inhibition rate of each group was calculated.
Inhibitory rate of tumor growth = [C(V1 - V0) - T(V1 - V0)]/C(V1 - V0)
Where C is the control group, T is the treated group, V1 is the volume before treatment (mm3), and V0 is the volume after treatment (mm3).
Tumor specimens were taken from areas next to the margin of the tumors as well as from more central areas. All the specimens were formalin-fixed, paraffin-embedded and pathologically diagnosed to be gastric carcinoma and evaluated using HE for conventional histological assessment. Histological characteristics were reviewed by two pathologists.
The tumor samples were cut into 4 μm thick slices and fixed in acetone. After washing in PBS, slices were incubated in 0.3% H2O2 solution at room temperature for 5 min. Slices were then incubated with anti-MMP-2 or anti-MMP-9 or anti-MVD or anti-VEGF monoclonal antibody at a 1:300 dilution at 4°C overnight. After washing in PBS, the second antibody, biotinylated anti-rat IgG, was added and the cells were incubated at room temperature for 1 h. After washing in PBS, avidin-biotin complex was added and then incubated at room temperature for 10 min. Diaminobenzidine was used as the chromogen. After 10 min, the brown color signifying the presence of antigen bound to antibodies was detected by light microscopy. Controls were prepared in the same manner as the experimental group, except for incubation with the primary antibody. The positive rate (PR) was calculated as follows: PR = (number of positive cells/total number of cells) × 100%.
The cytoplasm with MMP-2, MMP-9 and VEGF appeared as brown in color. Immunohistochemical staining was independently evaluated by two pathologists, who were blinded to the experimental data. Two hundred cells were chosen under the microscope to evaluate the stained cell number against the total cell number in the field. Based on the positive cell number, the criteria were set as follows: negative (-) having a positive cell number < 10%; (+) having 11%-50% positive cells; (++) having 51%-75% positive cells; and (+++) having > 75% positive cells. The results of staining of MMP-2, MMP-9 and VEGF were classified into negative (staining of ≤ 10% of cells) or positive (staining of > 10% of cells).
MVD-CD34 of tumor tissues were evaluated at low power field (× 40). The tissue sections were screened and five areas with the most intense neovascularization (hot spots) were selected. Microvessel counts of these areas were performed at high power field (× 200). Cases with discrepancies in scores between the two investigators were evaluated jointly to obtain a common consensus score. Any positively stained endothelial cell or endothelial cell cluster that was clearly separated from the adjacent microvessels, tumor cells and connective elements was counted as one microvessel, irrespective of the presence of a vessel lumen. An automated microvessel count/field was computed in each hot spot and the mean microvessel count of the 5 most vascularized areas was taken as the MVD-CD34, which was expressed as the absolute number of microvessels per 0.74 mm2 (× 200 field). The microvessel count that was higher than the median of microvessel count was taken as high MVD-CD34 and the microvessel count that was lower than the median of microvessel count was taken as low MVD-CD34.
All data are presented as mean% ± SD. Statistical analysis was carried out using ANOVA followed by Dunnett’s t-test, with P < 0.05 taken to indicate significance.
SGC7901 cells (1 × 109) were injected subcutaneously into the armpit of nude mice. Within 1 wk, visible tumors had developed at the injection sites. To determine the therapeutic effectiveness of LY294002, intratumoral injection of LY294002 was started after the volume of the implanted tumor reached 20 mm3, and was repeated every 2 d for a total of three times. As shown in Table 1, LY294002 markedly suppressed tumor growth compared with PBS alone (P < 0.01). No gross adverse effects, i.e. the loss of body weight, were observed during the experimental periods. Moreover, LY294002 inhibited the proliferation of the implanted tumor of human SGC7901 cells in nude mice in a time-dependent fashion. The inhibition rate of the tumors was 11.31% ± 13% on day 5, 29.4% ± 1.47% on day 10 and reached 36.7% ± 2.12% on day 15.
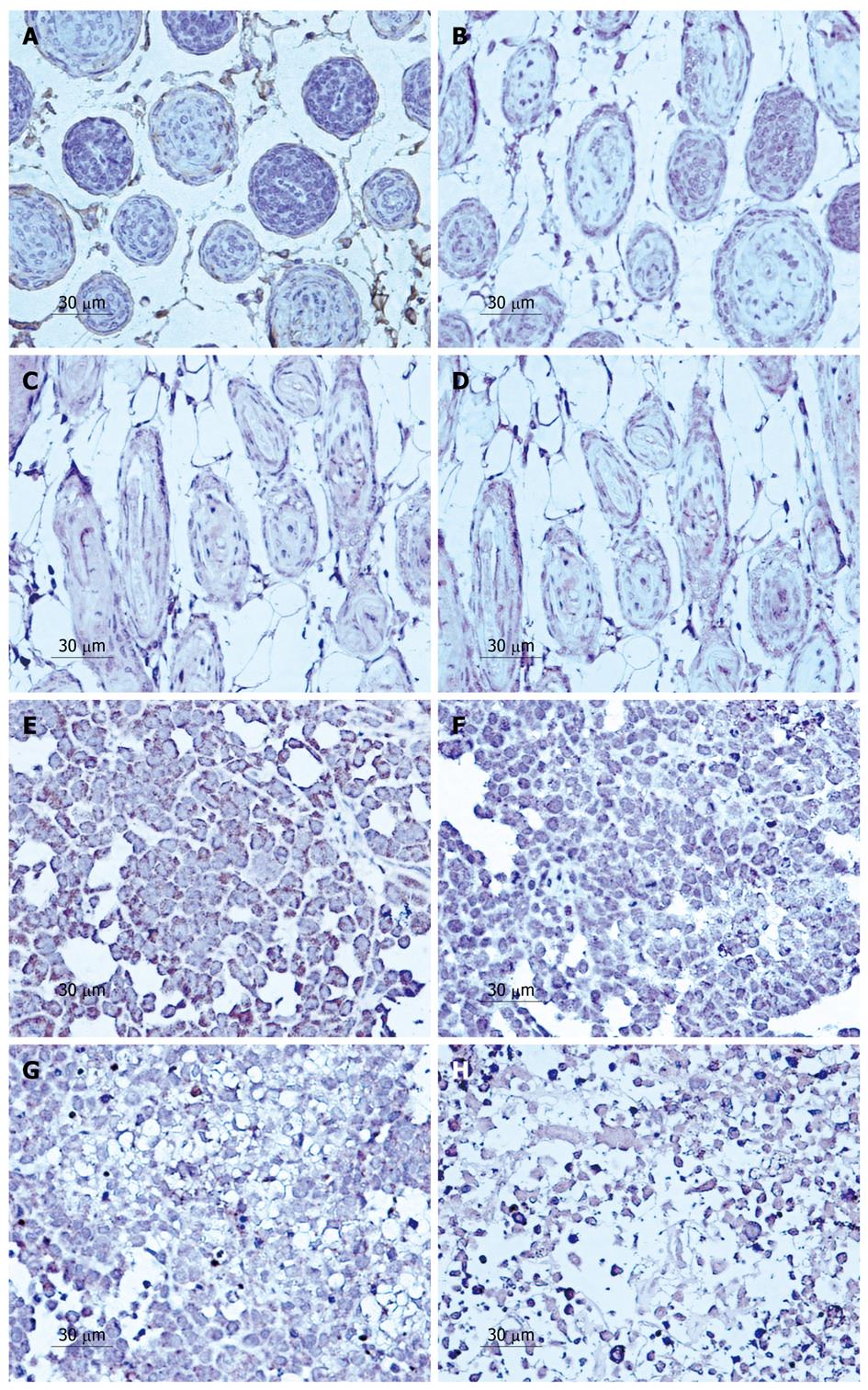
Treatment with LY294002 for 5, 10 and 15 d in SGC7901 cells produced intensive HE staining indicating apoptosis. A significant increase in apoptosis was observed along with the time that tumors were treated (Figure 1). After 5 d, the rate of inhibition reached 23.51% ± 3.11%. The rate of inhibition rose when the experimental time increased, reaching 43.20% ± 3.27% on day 10 and 63.28% ± 2.10% on day 15 after LY294002 treatment. All the results indicated that LY294002 induced apoptosis (Figure 1E-H).
Similarly, in the lymphatic vessels, the effect of the drug increased with time. The invasion rate of the tumor cells in the lymphatic vessels decreased from 75.69% ± 3.5% to 63.78% ± 4.3%, 45.62% ± 3.1% and 28.17% ± 2.7% at 5, 10 and 15 d, respectively, after treatment with LY294002 (Figure 1A-D).
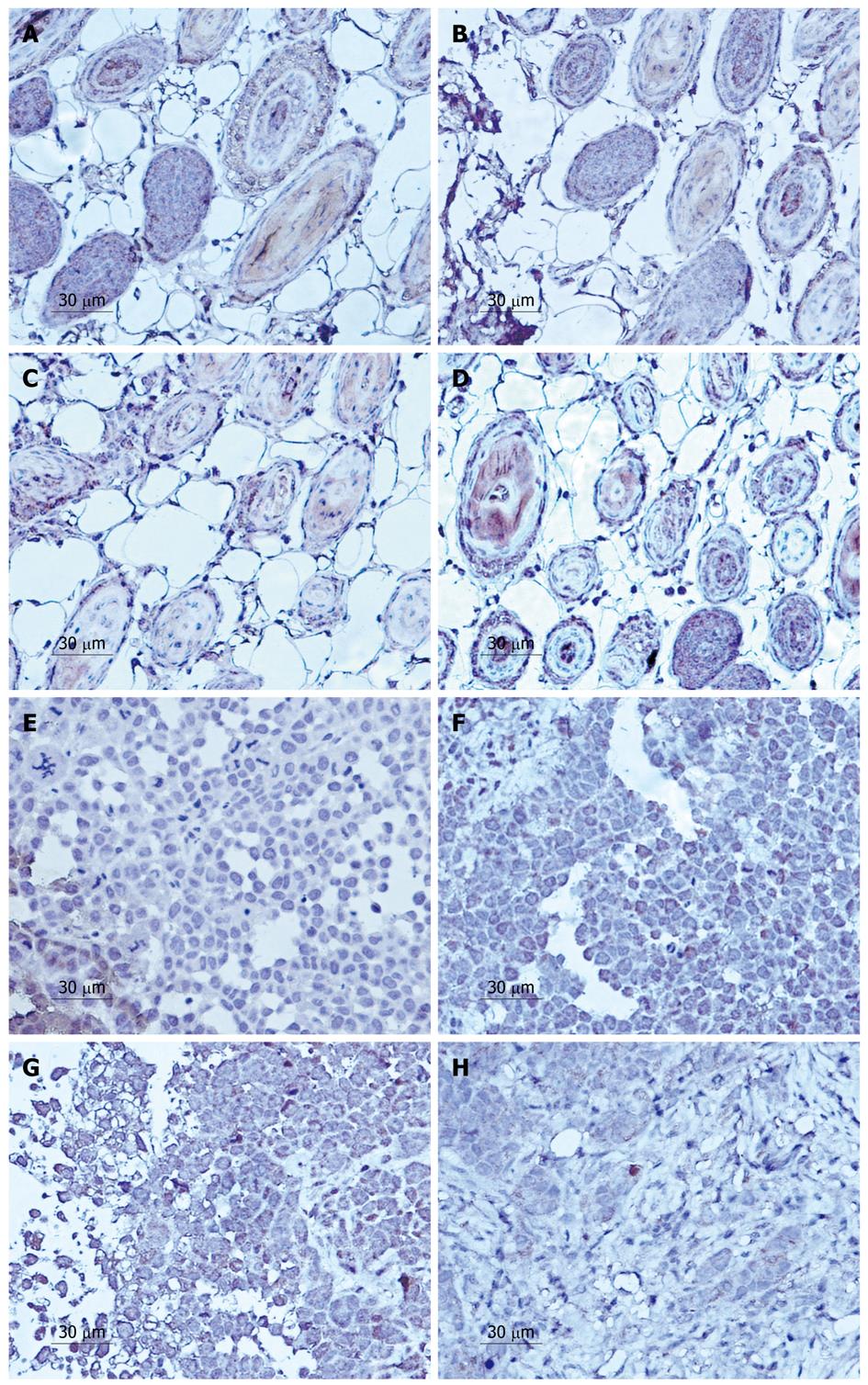
Positive staining was distributed in the cell membrane and cytoplasm (Figures 2 and 3). The PR of MMP-2 protein downregulated was from 71.4% ± 1.6% in the control group to 47.9% ± 0.7%, 31.9% ± 0.9% and 7.9% ± 0.7% at 5, 10 and 15 d, respectively, after treatment with LY294002 for (Figure 2E-H). Significant differences in the expression were observed between the 500 μmol/L LY249002 group and the control group at every time point (P < 0.05). Similarly, the expression of MMP-9 decreased from 49.4% ± 1.5% to 36.9% ± 0.4%, 23.5% ± 0.9%, 7.7% ± 0.6%, respectively (Figure 3E-H).
In the lymphatic vessels, we found that the expression of MMP-2 in the tumor cells also decreased from 59.23% ± 6.2% to 45.46% ± 7.3%, 32.14% ± 1.9%, 14.01% ± 3.9% at the various time points (Figure 2A-D). Interestingly, MMP-9 shared the same characteristics in expression as that of MMP-2, which fell from 39.95% ± 5.7% to 25.32% ± 6.5%, 12.84% ± 2.8%, 4.01% ± 5.9% (Figure 3A-D).
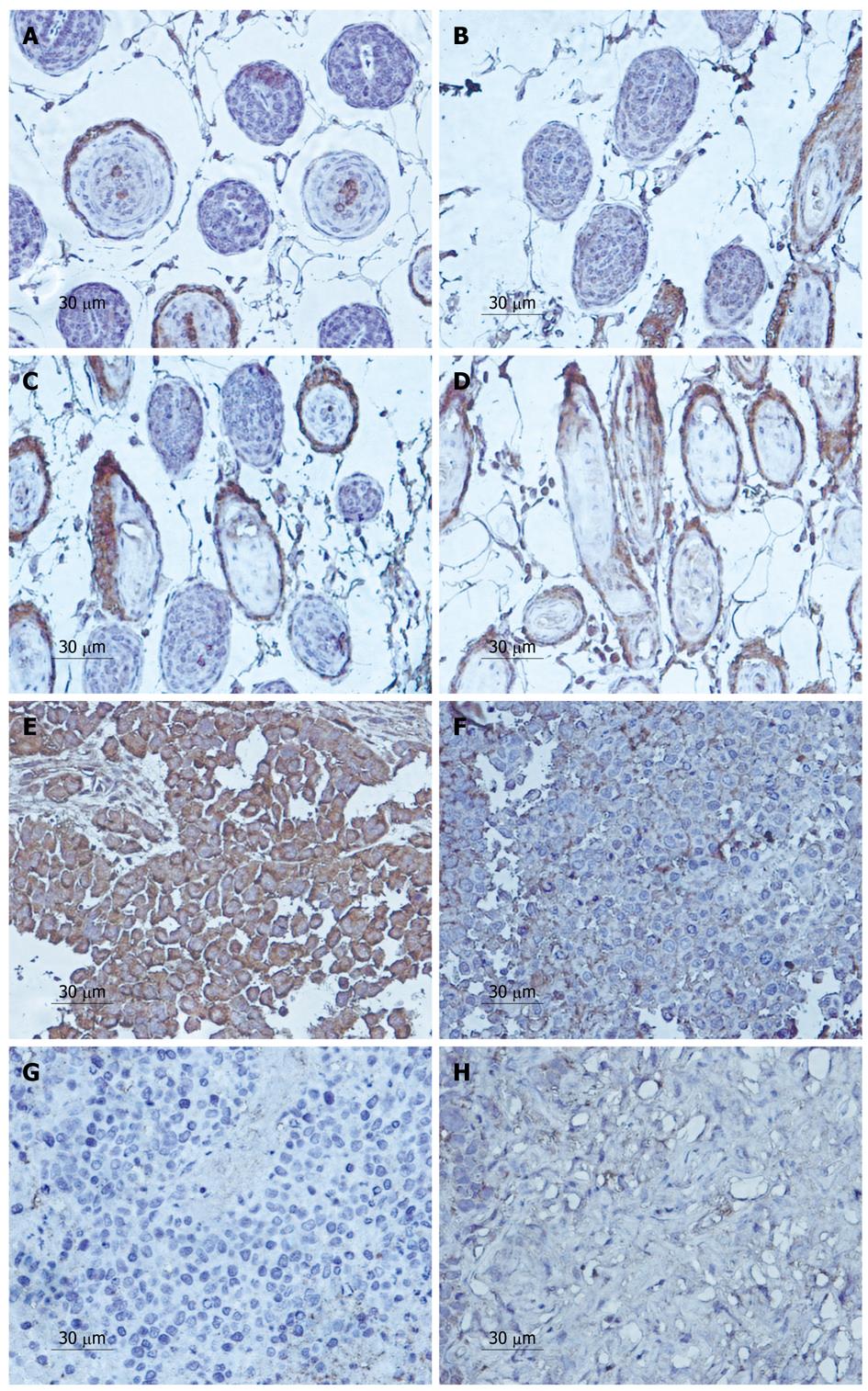
Positively stained particles of MVD-CD34 were mainly distributed in the plasmalemma of cells, though some were expressed in the cytoplasm (Figure 4). The mean MVD was much lower in the tumors treated with LY294002 than in the control group.
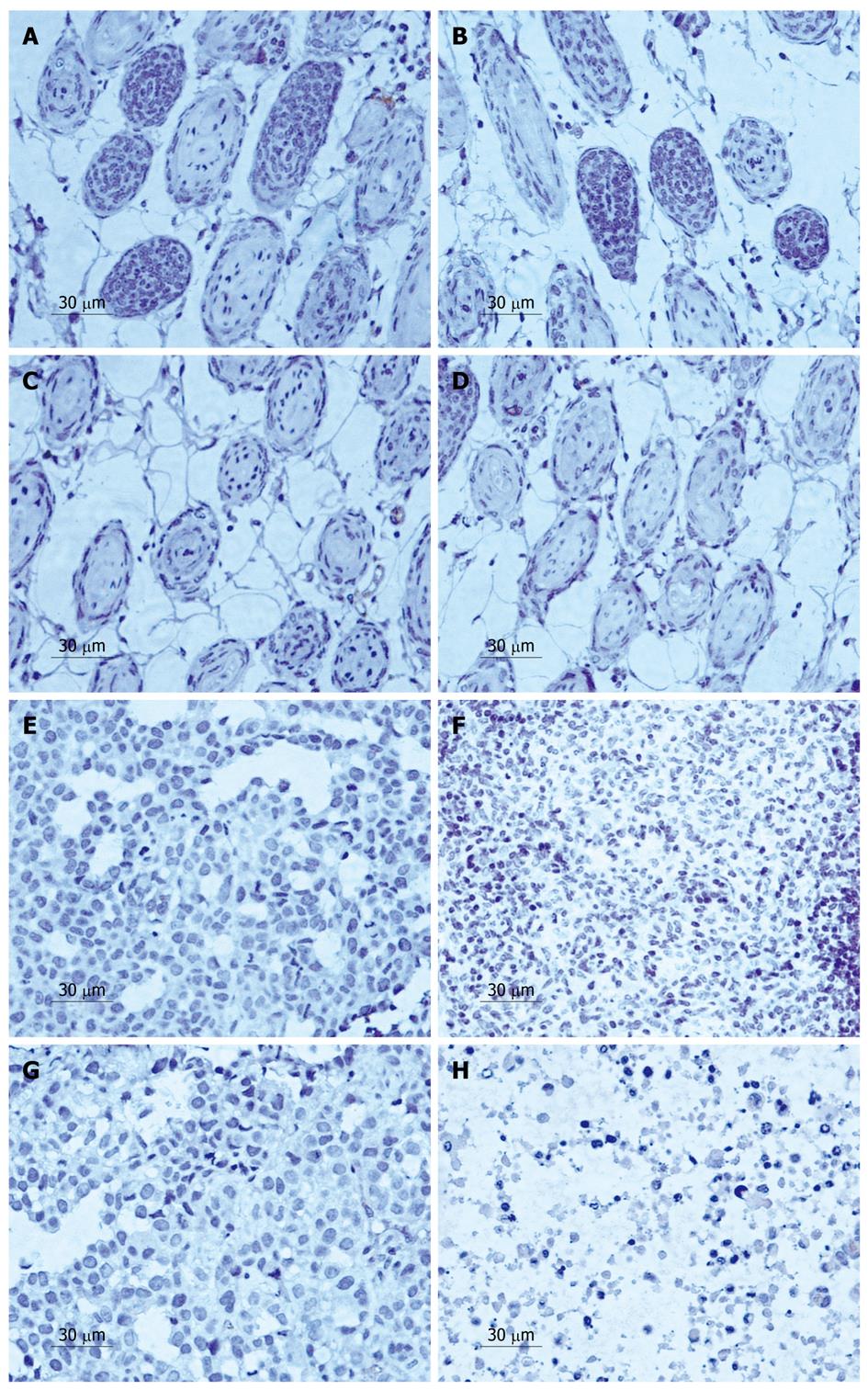
According to the PR of MVD protein of the control group and the experimental group after treatment with LY294002 5, 10, 15 d, the expression of MVD was downregulated from 51.2% ± 3.1% to 41.9% ± 1.5%, 30.9% ± 1.7% and 14.9% ± 0.8% after 5, 10 and 15 d, respectively (Figure 4E-H). A significant difference in positive expression was observed between the 500 μmol/L LY294002 group and the control group at every time point (P < 0.05). Similarly, the expression of VEGF decreased from 47.2% ± 3.1% to 25.9% ± 0.5%, 18.6% ± 1.2% and 5.1% ± 0.9%, respectively (Figure 5E-H).
In the vascular area, we found that the effect of the agent increased with time, and the MVD of the tumor cells decreased from 37.65% ± 2.7% to 27.24% ± 5.3%, 10.64% ± 3.5%, 5.26% ± 1.3% at 5, 10 and 15 d, respectively (Figure 4A-D). The VEGF expression was also downregulated from 34.25% ± 1.7% to 25.21% ± 2.3%, 11.34% ± 5.1% and 3.26% ± 0.3%, respectively (Figure 5A-D).
Currently only a few chemotherapeutic drugs are effective for the treatment of human gastric carcinoma[13] and there is an increasing interest in the use of drugs to prevent its occurrence or invasiveness. The lipid kinase PI3K is a proto-oncogene that generates 3’-phosphoinositides at the cell membrane. The best-characterized inhibitors of PI3K are LY294002 and wortmannin. LY294002 effectively inhibits the growth of many types of tumor cells in vitro and in vivo, via the inhibition of PI3K and downstream components of the pathway[14-17]. It is possible that LY294002 inhibits proliferation and induces apoptosis in SGC7901 cells through inhibiting class I PI3K. However, the downstream molecules involved in the apoptotic death of tumor cells following inhibition of PI3K/Akt by LY294002 remain to be identified[1,18].
Tumor metastasis involves a series of complex processes in which many gene products take part in regulating MMPs, which play an important role in breaking the extracellular matrix, are overexpressed in malignant tumors and are believed to contribute to tumor proliferation, invasion, and metastasis[19]. Among the MMPs, MMP-2 and MMP-9 are closely related to the metastasis of tumors, and have been specifically considered to be important factors in facilitating lymphatic invasion and metastases in gastric carcinoma[20-22].
MVD is a quantitative index for carcinoma angiogenesis, which indicates the regulation of the progress and metastasis of primary gastric carcinoma, and can therefore serve as a marker for carcinoma prognosis[23-25]. VEGF is one of the agents accelerating the formation of blood vessels, and plays a vital part in tumor-associated microvascular invasion[26-28]. It has been found that tumor metastasis is speeded up by VEGF, which is highly expressed in gastric carcinoma, thus may be used as an index for poor prognosis of gastric carcinoma[29-31].
In the present study, there was a significant difference in the expression of MMP-2, MMP-9 and VEGF, and in MVD between the transplanted gastric tumor tissues treated by the class I PI3K inhibitor LY249002 and that of the control group (P < 0.05), indicating that LY249002 may inhibit the gene expression of MMP-2, MMP-9, MVD, and VEGF. We also showed that LY294002 reduced the viability and induced apoptosis in the implanted tumor of human SGC7901 cells, thus demonstrating the cytotoxic effects of LY294002. Both the in vitro invasion assay and the in vivo nude mice assay suggested that LY249002 had the potential to inhibit the invasion and metastasis of gastric cancer. This may be the result of the decrease of the expression of MMP-2, MMP-9, MVD and VEGF together with the cytotoxicity towards the tumor cells, both induced by LY294002. Moreover, no gross adverse effects, i.e. loss of body weight, were observed during our experimental periods. These findings suggest that inhibition of the class I PI3K signaling pathway is a potent and safe strategy for managing gastric cancers, and further indicated that development of LY249002-based therapies may be an emerging approach for the next step in gastric cancer management.
The phosphatidylinositol 3-kinase (PI3K) pathway plays a central role in the regulation of cell proliferation, growth, differentiation, and survival. Dysregulation of this pathway is frequently observed in a variety of tumors, including brain tumors and breast, ovarian and other carcinomas. Currently only a few chemotherapeutic drugs are effective for the treatment of human gastric carcinoma and there is an increasing interest in the use of drugs to prevent its occurrence or invasiveness.
LY294002 is a specific inhibitor of class I PI3K. The antitumor activity of LY294002 may be related to the induction of apoptosis of tumor cells, but the precise mechanism of its antitumor activity is not well understood.
The research indicated that development of LY249002-based therapies may be an emerging approach for the next step in gastric cancer management.
Matrix metalloproteinases (MMPs) are a family of structurally related zinc-endopeptidases. MMP-2 contributes to cell proliferation, migration, and matrix invasion in a number of cell types such as tumor cells and fibroblasts MMP-9 is directly involved in tumor metastasis, and more recently MMP-9 activity has been linked with the process of tumor cell invasiveness. High MVD is an indirect measure of tumor aggressiveness. Vascular endothelial growth factor is a potent regulator of placental vascular function. Endothelial dysfunction is a key factor associated with preeclampsia.
Overall, the experiment was well performed and the authors have presented interesting data to provide a mechanistic insight into the antitumor effect of LY294002.
| 1. | Xing CG, Zhu BS, Liu HH, Lin F, Yao HH, Liang ZQ, Qin ZH. LY294002 induces p53-dependent apoptosis of SGC7901 gastric cancer cells. Acta Pharmacol Sin. 2008;29:489-498. |
| 2. | Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS, Lin JT, Inoue H, Chen GH. Helicobacter pylori promote gastric cancer cells invasion through a NF-kappaB and COX-2-mediated pathway. World J Gastroenterol. 2005;11:3197-3203. |
| 3. | Lazăr D, Tăban S, Raica M, Sporea I, Cornianu M, Goldiş A, Vernic C. Immunohistochemical evaluation of the tumor neoangiogenesis as a prognostic factor for gastric cancers. Rom J Morphol Embryol. 2008;49:137-148. |
| 4. | Wang J, Tian XF, Wang S, Ma LF, Yao JH. Correlation between expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and angiogenesis in gastric cancer. Chin J Cancer Res. 2005;17:283-287. |
| 5. | Sun WH, Sun YL, Fang RN, Shao Y, Xu HC, Xue QP, Ding GX, Cheng YL. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in gastric carcinoma and its correlation with angiogenesis. Jpn J Clin Oncol. 2005;35:707-713. |
| 6. | Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med. 2003;81:20-31. |
| 7. | Vacca A, Ria R, Ribatti D, Semeraro F, Djonov V, Di Raimondo F, Dammacco F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003;88:176-185. |
| 8. | Vogt PK. PI 3-kinase, mTOR, protein synthesis and cancer. Trends Mol Med. 2001;7:482-484. |
| 9. | Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79-85. |
| 10. | Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3'-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742-2746. |
| 11. | Neve RM, Holbro T, Hynes NE. Distinct roles for phosphoinositide 3-kinase, mitogen-activated protein kinase and p38 MAPK in mediating cell cycle progression of breast cancer cells. Oncogene. 2002;21:4567-4576. |
| 12. | Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426-7429. |
| 13. | Zhou HB, Chen JM, Cai JT, Du Q, Wu CN. Anticancer activity of genistein on implanted tumor of human SG7901 cells in nude mice. World J Gastroenterol. 2008;14:627-631. |
| 14. | Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3'-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957-1963. |
| 15. | Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127-3134. |
| 16. | Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin Cancer Res. 2000;6:880-886. |
| 17. | Crighton D, Ryan KM. Splicing DNA-damage responses to tumour cell death. Biochim Biophys Acta. 2004;1705:3-15. |
| 18. | Xing C, Zhu B, Liu H, Yao H, Zhang L. Class I phosphatidylinositol 3-kinase inhibitor LY294002 activates autophagy and induces apoptosis through p53 pathway in gastric cancer cell line SGC7901. Acta Biochim Biophys Sin (Shanghai). 2008;40:194-201. |
| 19. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. |
| 20. | Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Relationship between biologic behavior and phenotypic expression in intramucosal gastric carcinomas. Hum Pathol. 2002;33:80-86. |
| 21. | Cai H, Kong ZR, Chen HM. [Matrix metalloproteinase-2 and angiogenesis in gastric cancer]. Aizheng. 2002;21:25-28. |
| 22. | Takahashi Y, Kitadai Y, Ellis LM, Bucana CD, Fidler IJ, Mai M. Multiparametric in situ mRNA hybridization analysis of gastric biopsies predicts lymph node metastasis in patients with gastric carcinoma. Jpn J Cancer Res. 2002;93:1258-1265. |
| 23. | Zhang Y, Wu XH, Cao GH, Li S. [Relationship between expression of matrix metalloproteinase-9 (MMP-9) and angiogenesis in renal cell carcinoma]. Aizheng. 2004;23:326-329. |
| 24. | Offersen BV, Borre M, Overgaard J. Quantification of angiogenesis as a prognostic marker in human carcinomas: a critical evaluation of histopathological methods for estimation of vascular density. Eur J Cancer. 2003;39:881-890. |
| 25. | van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426-437. |
| 26. | Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913-1926. |
| 27. | Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10-14. |
| 28. | Bellamy WT. Expression of vascular endothelial growth factor and its receptors in multiple myeloma and other hematopoietic malignancies. Semin Oncol. 2001;28:551-559. |
| 29. | Tian X, Song S, Wu J, Meng L, Dong Z, Shou C. Vascular endothelial growth factor: acting as an autocrine growth factor for human gastric adenocarcinoma cell MGC803. Biochem Biophys Res Commun. 2001;286:505-512. |
| 30. | Mao ZB, Xiao MB, Huang JF, Ni HB, Ni RZ, Wei Q, Zhang H. Expression of VEGF and its signification in serum of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:1220-1221. |
| 31. | Lou G, Gao Y, Ning XM, Zhang QF. Expression and correlation of CD44v6, vascular endothelial growth factor, matrix metalloproteinase-2, and matrix metalloproteinase-9 in Krukenberg tumor. World J Gastroenterol. 2005;11:5032-5036. |
Peer reviewer: Dr. Wang-Xue Chen, Institute for Biological Sciences, National Research Council Canada, 100 Sussex Drive, Room 3100, Ottawa, Ontario K1A 0R6, Canada
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM