Published online Aug 28, 2009. doi: 10.3748/wjg.15.4055
Revised: July 31, 2009
Accepted: August 7, 2009
Published online: August 28, 2009
AIM: To analyze the differences and relevance of Yes-associated protein (YAP) and survivin, and to explore the correlation and significance of their expression in gastric carcinoma and precancerous lesions.
METHODS: The PV9000 immunohistochemical method was used to detect the expression of YAP and survivin in 98 cases of normal gastric mucosa, 58 intestinal metaplasia (IM), 32 dysplasia and 98 gastric carcinoma.
RESULTS: The positive rates of YAP in dysplasia (37.5%) and gastric carcinoma (48.0%) were significantly higher than that in normal gastric mucosa (13.3%), P < 0.01. The positive rates of survivin in IM (53.4%), dysplasia (59.4%) and gastric carcinoma (65.3%) were significantly higher than in normal gastric mucosa (11.2%), P < 0.01. Survivin expression gradually increased from 41.7% in well differentiated adenocarcinoma through 58.3% in moderately differentiated adenocarcinoma to 75.6% in poorly differentiated adenocarcinoma, with significant Rank correlation, rk = 0.279, P < 0.01. The positive rate of survivin in gastric carcinoma of diffused type (74.6%) was significantly higher than that in intestinal type (51.3%), P < 0.05. In gastric carcinoma with lymph node metastasis (76.9%), the positive rate of survivin was significantly higher than that in the group without lymph node metastasis (41.2%), P < 0.01. In 98 cases of gastric carcinoma, the expression of YAP and of survivin were positively correlated, rk = 0.246, P < 0.01.
CONCLUSION: YAP may play an important role as a carcinogenic factor and may induce survivin expression. Detecting both markers together may help in early diagnosis of gastric carcinoma.
- Citation: Da CL, Xin Y, Zhao J, Luo XD. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World J Gastroenterol 2009; 15(32): 4055-4061
- URL: https://www.wjgnet.com/1007-9327/full/v15/i32/4055.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4055
Yes-associated protein (YAP) is a type of cellular adaptor protein and transcriptional co-activator, which was initially isolated by Sudol et al[1] in 1994, as a result of its binding to the Src family member non-receptor tyrosine kinase YES (Yes kinase-associated protein). In biological conditions, YAP was described as a target of the Hippo (Hpo)-Salvador-Warts pathway and was phosphorylated by the pathway to negatively regulate growth by simultaneously inhibiting proliferation and promoting apoptosis. In recent years, some investigators have found YAP to be overexpressed and highly activated in hepatic cancers and mammary cancers[2–4], suggesting its carcinogenicity. Survivin is a new member of the inhibitor of apoptotic protein (IAP) family, and was initially cloned by the cDNA of effector cell protease receptor-1 in the human genomic library in 1997[5]. Many investigations have found survivin to be overexpressed in most common tumors, but almost never in normal tissues[6]. The overexpression of survivin was closely related to tumorigenesis and progression, and was one of the strongest apoptotic inhibitors identified. In tumors, lack of, or mutation of, any factor(s) in the Hpo signaling pathway can lead to dephosphorylation and activation of YAP, which then induces a high expression of Ki67, c-myc, SOX4, H19, AFP, BIRC5/survivin, BIRC2/cIAP1 and other cellular proliferation-related genes and inhibitors of apoptosis. Of note is the massive induction (30-fold) of BIRC5/survivin, leading to breakdown in the balance of cellular proliferation and apoptosis, and an increase in the occurrence and development of tumors[7].
Gastric cancer is one of the malignant diseases with the highest incidence and mortality rates, but its pathophysiology remains to be clarified. We measured the expression level of YAP and survivin in normal gastric mucosa, precancerous lesions and gastric carcinoma using an immunohistochemical (IHC) method in order to analyze the significance and correlations of these two factors with gastric tumorigenesis.
We collected gastric carcinoma specimens from the First Affiliated Hospital of China Medical University: 98 cases of gastric carcinoma, including 29 cases of early gastric carcinoma (EGC) and 69 cases of advanced gastric carcinoma (AGC), with matched normal gastric mucosa, 58 cases of intestinal metaplasia (IM), and 32 cases of dysplasia (DYS). There were 66 males and 32 females, mean age 60 years. Gross types were as follows: EGC cases: 18 cases of type I + IIc, 10 cases of type III, one case of extensive superficial type; AGC cases: seven cases of Borrmann I + II, 62 cases of Borrmann III + IV. According to the World Health Organization histological classification of GC, the 98 cases were classified as follows: two papillary adenocarcinoma, 12 well differentiated adenocarcinoma, 25 moderately differentiated adenocarcinoma, 41 poorly differentiated adenocarcinoma, two undifferentiated adenocarcinoma, seven signet ring cell carcinomas and nine mucinous adenocarcinoma.
Samples were fixed in 10% formalin, embedded in paraffin, cut into 4 μm thick sections and constructed in blocks for tissue microarray. All the samples were evaluated by two experienced pathologists for diagnosis.
Expression of YAP and survivin in gastric carcinomas, precancerous lesions and normal gastric mucosa were detected using an IHC method. A PV-9000 kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Company. Anti-human rabbit YAP polyclonal antibody was purchased from the Cell Signaling Technology Company (working dilution 1:25). Anti-human rabbit polyclonal antibody survivin (ready to use) was purchased from Fuzhou Maixin Company (China). All procedures were implemented according to the manufacturer’s instructions. For negative controls, sections were treated with 0.01 mol/L phosphate-buffered saline instead of primary antibodies.
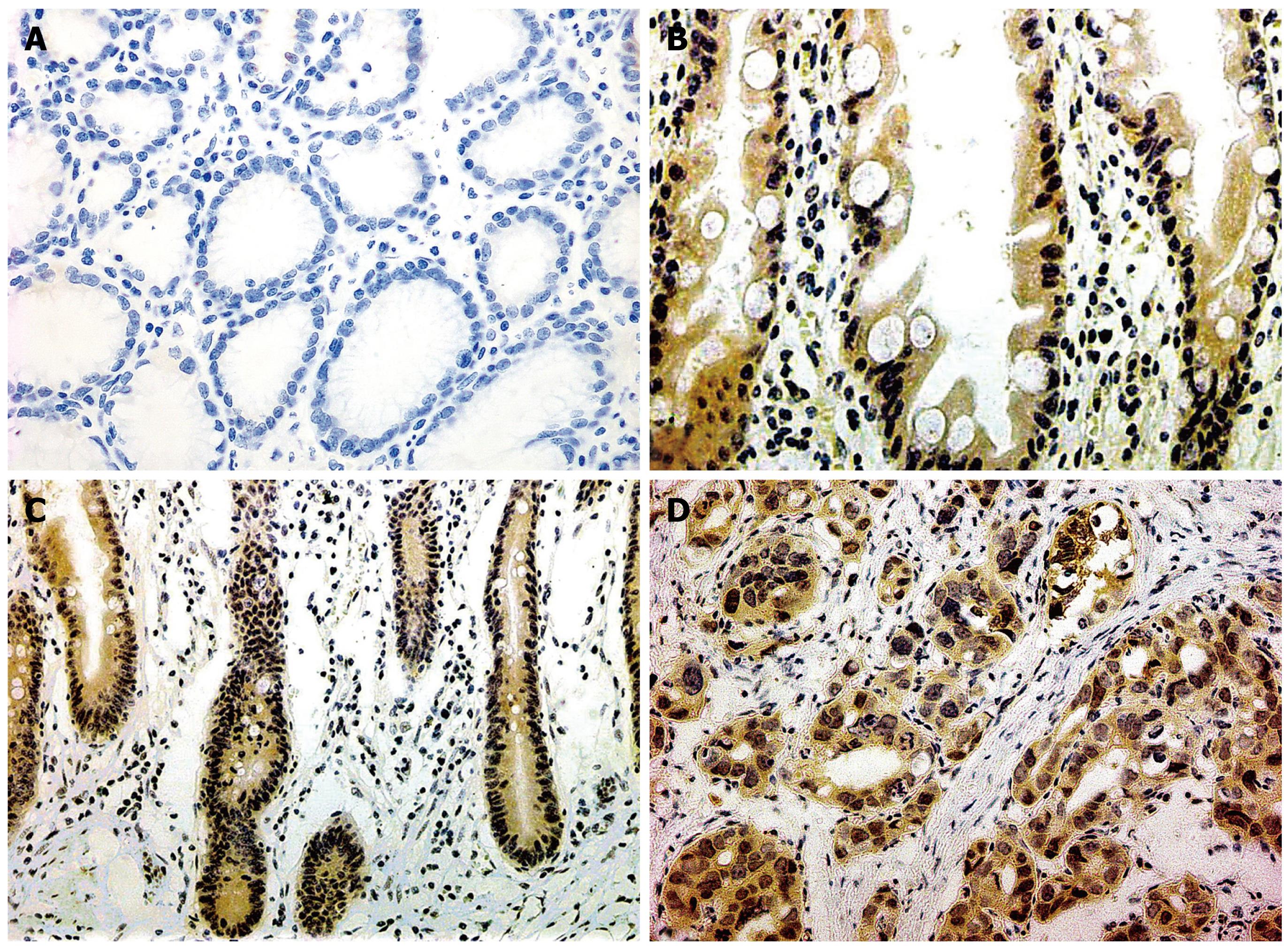
YAP was specifically located in the cytoplasm and nucleus of carcinoma cells; survivin was specifically located in the cytoplasm of carcinoma cells. Staining intensity (A) was classified as 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The percentage of positive cells (B) examined in 200 cells were divided into 0 (< 5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%) and 4 (> 75%). According to the product of A and B, the IHC result was classified as 0, negative (-); 1-4, weakly positive (+); 5-8, moderately positive (++) and 9-12, strongly positive (+++).
Statistical analysis was performed using SPSS 11.5 Package, χ2 test, Fisher’s exact test and Kendall’s tau-b test were used to differentiate the rates of different groups and test the correlation between the two factors. P < 0.05 was considered statistically significant.
The positive rates of YAP presence in dysplasia (37.5%) and gastric carcinoma (48.0%) were significantly higher than that in normal gastric mucosa (13.3%), P < 0.01; there was no statistically significant difference between YAP expression in the normal gastric mucosa and IM (16/58, 27.6%), dysplasia and gastric carcinoma, P > 0.05. YAP expression showed an increasing trend from well differentiated adenocarcinoma (4/12, 33.3%), through moderately differentiated adenocarcinoma (11/25, 44.0%) to poorly differentiated adenocarcinoma (24/41, 58.5%), although without significant Rank correlation. The positive rate of YAP expression showed an increasing trend from gastric carcinoma without lymph node metastasis (5/17, 29.4%), to gastric carcinoma with lymph node metastasis (24/52, 46.2%), though without statistical significance, P > 0.05. There was no significant correlation of the expression of YAP with patients’ gender, age, Borrmann’s classification of gastric carcinoma or Lauren classification, P > 0.05 (Tables 1 and 2, Figure 1).
| Groups | n | YAP expression | Positive (%) | P | ||||
| - | + | ++ | +++ | |||||
| Sex | 0.309 | |||||||
| Male | 66 | 31 | 22 | 8 | 5 | 53.0 | ||
| Female | 32 | 20 | 8 | 4 | 0 | 37.5 | ||
| Age | 0.304 | |||||||
| < 60 | 54 | 30 | 15 | 8 | 1 | 45.5 | ||
| ≥ 60 | 44 | 21 | 15 | 4 | 4 | 51.2 | ||
| Gross type | ||||||||
| EGC | 0.937 | |||||||
| Type I + IIc | 18 | 6 | 8 | 3 | 1 | 66.7 | ||
| Type III | 10 | 4 | 3 | 2 | 1 | 60.0 | ||
| AGC | 0.074 | |||||||
| Type BorI + II | 7 | 2 | 2 | 2 | 1 | 71.4 | ||
| Type BorIII + IV | 62 | 38 | 17 | 5 | 2 | 38.7 | ||
| WHO’s histological types | rk = 0.181 | 0.079 | ||||||
| Well-diff. ade. | 12 | 8 | 4 | 0 | 0 | 33.3 | 0.635a | |
| Moderately-diff. ade. | 25 | 14 | 6 | 3 | 2 | 44.0 | 0.673b | |
| Poorly-diff. ade. | 41 | 17 | 15 | 6 | 3 | 58.5 | 0.406c | |
| Undiff. ade. | 2 | 2 | 0 | 0 | 0 | 0.0 | ||
| Papillary ade. | 2 | 0 | 1 | 1 | 0 | 100.0 | ||
| Signet ring cell carcinoma | 7 | 4 | 2 | 1 | 0 | 42.9 | ||
| Mucinous ade. | 9 | 6 | 2 | 1 | 0 | 33.3 | ||
| Lauren types | 0.669 | |||||||
| Intestinal type carcinoma | 39 | 23 | 11 | 4 | 1 | 41.0 | ||
| Diffused type carcinoma | 59 | 28 | 19 | 8 | 4 | 52.5 | ||
| Lymph node metastasis | 0.602 | |||||||
| Yes | 52 | 28 | 16 | 5 | 3 | 46.2 | ||
| No | 17 | 12 | 3 | 2 | 0 | 29.4 | ||
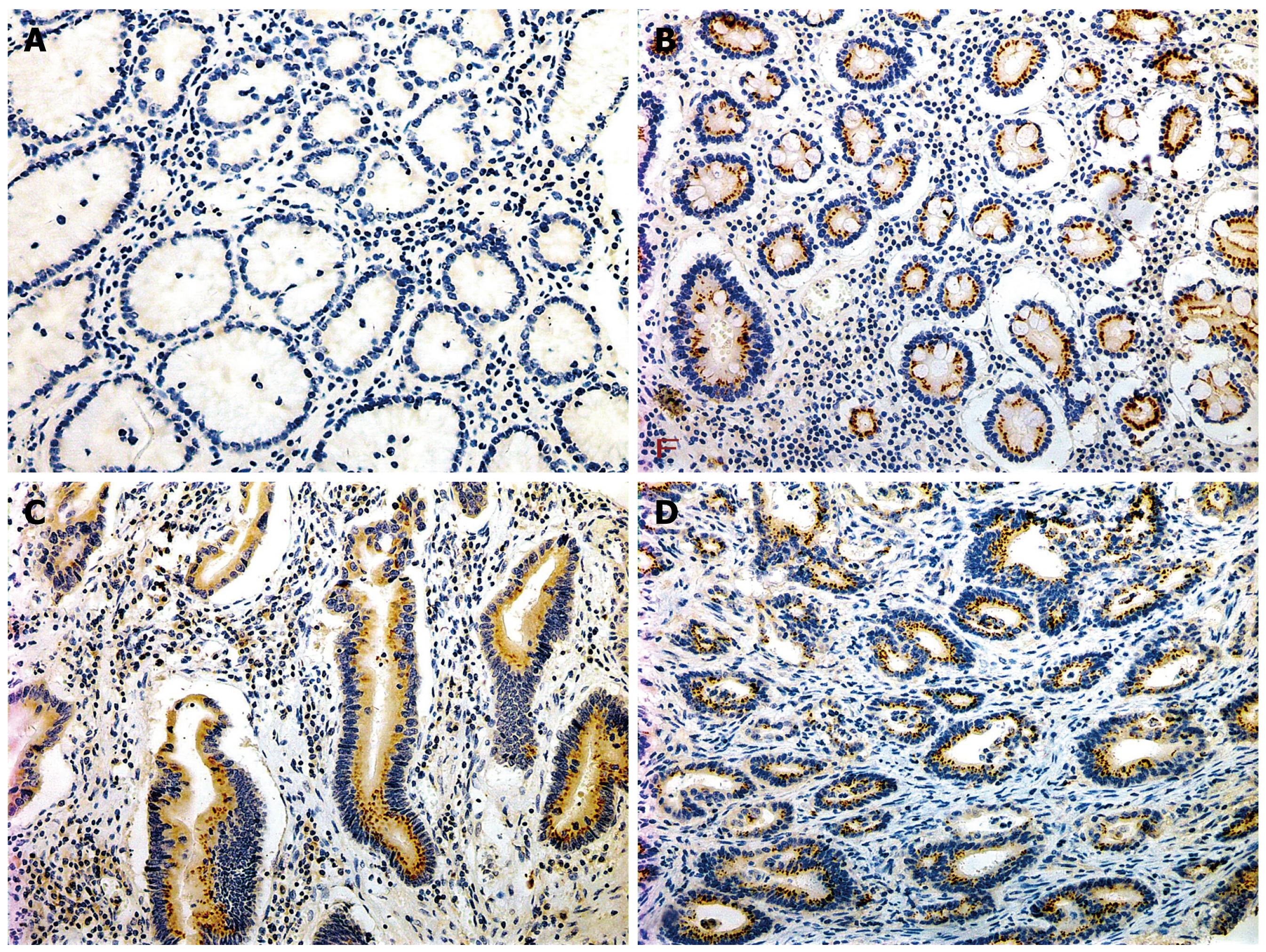
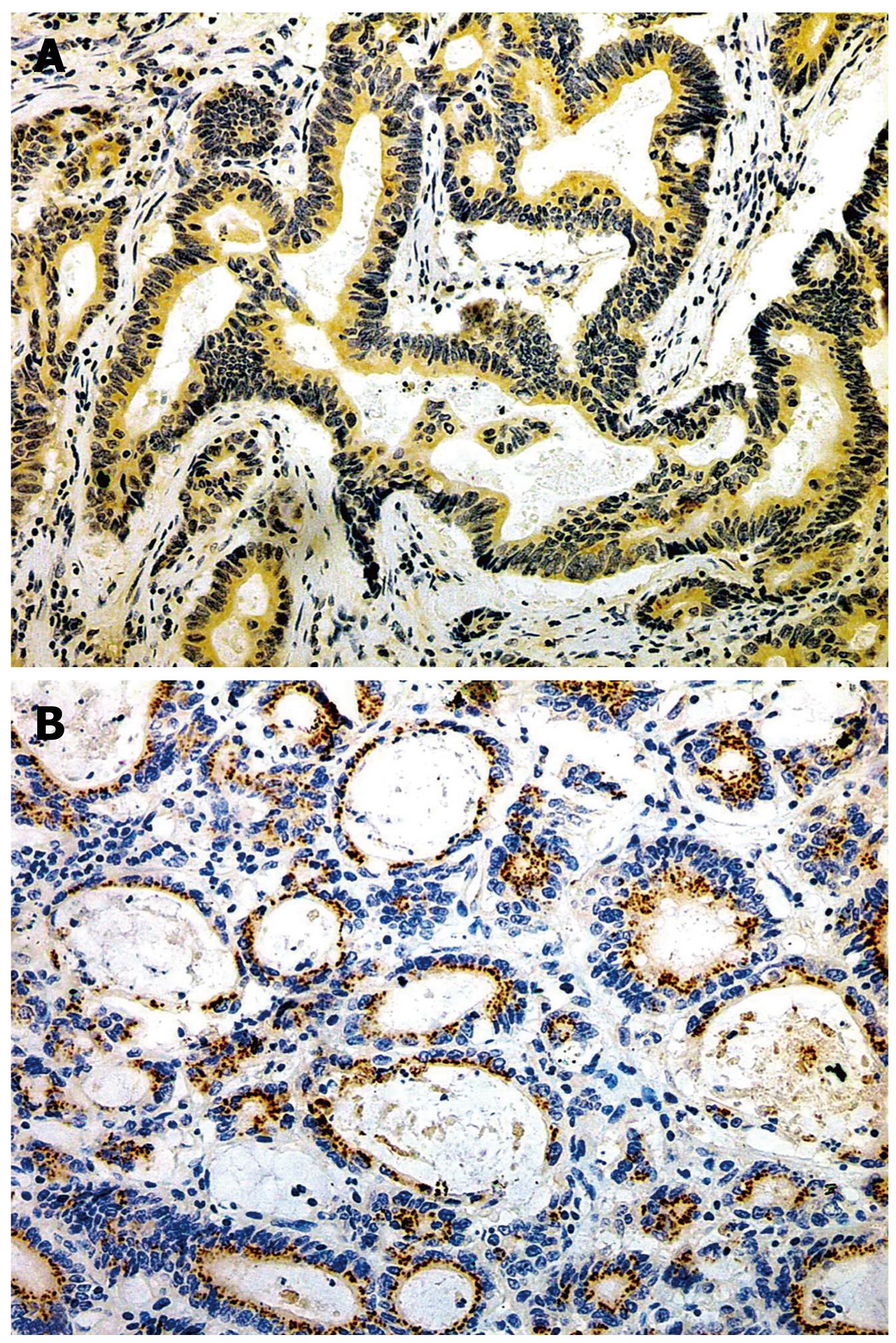
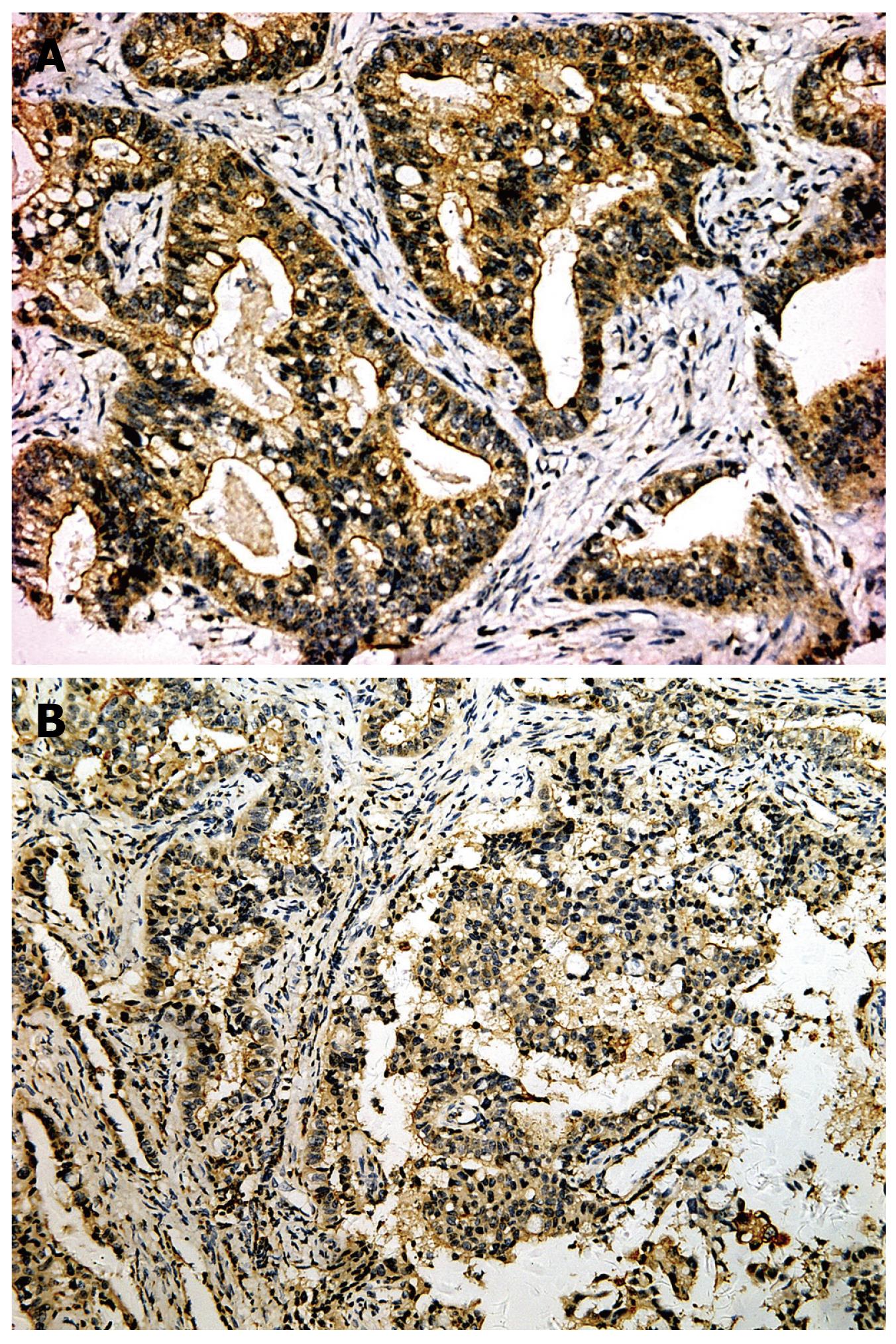
The positive rates of survivin in IM (53.4%), dysplasia (59.4%) and gastric carcinoma (65.3%) were significantly higher than that in normal gastric mucosa (11.2%), P < 0.01. The expression level gradually increased from well differentiated adenocarcinoma (41.7%), through moderately differentiated adenocarcinoma (58.3%) to poorly differentiated adenocarcinoma (75.6%), with significant Rank correlation, rk = 0.279, P < 0.01. The positive rate of survivin in gastric carcinoma of diffused type (74.6%) was significantly higher than that in intestinal type (51.3%), P < 0.05. In gastric carcinoma with lymph node metastasis (76.9%), the positive rate of survivin was significantly higher than that in the group without lymph node metastasis (41.2%), P < 0.01, There was no relationship between gastric carcinoma and sex, age and gross type of carcinoma (Tables 3 and 4, Figures 2, 3, 4).
| Groups | n | Survivin expression | Positive (%) | χ² | P | |||
| - | + | ++ | +++ | |||||
| Sex | 4.87 | 0.172 | ||||||
| Male | 66 | 20 | 27 | 14 | 5 | 69.7 | ||
| Female | 32 | 14 | 7 | 6 | 5 | 56.2 | ||
| Age | 2.74 | 0.434 | ||||||
| < 60 | 54 | 20 | 15 | 13 | 6 | 63.0 | ||
| ≥ 60 | 44 | 14 | 19 | 7 | 4 | 68.2 | ||
| Gross type | ||||||||
| EGC | 0.3101 | |||||||
| Type I + IIc | 18 | 5 | 7 | 3 | 3 | 72.2 | ||
| Type III | 10 | 6 | 1 | 2 | 1 | 40.0 | ||
| AGC | 0.6961 | |||||||
| Type BorI+ II | 7 | 3 | 2 | 1 | 1 | 57.1 | ||
| Type BorIII+ IV | 62 | 19 | 24 | 14 | 5 | 69.4 | ||
| WHO’s histological types | rk = 0.279 | 0.006 | ||||||
| Well-diff. ade. | 12 | 7 | 4 | 1 | 0 | 41.7 | 0.8241a | |
| Moderately-diff. ade. | 25 | 11 | 10 | 2 | 2 | 56.0 | 0.2231b | |
| Poorly-diff. ade. | 41 | 10 | 16 | 10 | 5 | 75.6 | 0.1491c | |
| Undiff. ade. | 2 | 1 | 1 | 0 | 0 | 50.0 | ||
| Papillary ade. | 2 | 1 | 0 | 1 | 0 | 50.0 | ||
| Signet ring cell carcinoma | 7 | 1 | 2 | 3 | 1 | 85.7 | ||
| Mucinous ade. | 9 | 3 | 1 | 3 | 2 | 66.7 | ||
| Lauren types | 8.61 | 0.035 | ||||||
| Intestinal type carcinoma | 39 | 19 | 14 | 4 | 2 | 51.3 | ||
| Diffused type carcinoma | 59 | 15 | 20 | 16 | 8 | 74.6 | ||
| Lymph node metastasis | 0.0051 | |||||||
| Yes | 52 | 12 | 19 | 15 | 6 | 76.9 | ||
| No | 17 | 10 | 7 | 0 | 0 | 41.2 | ||
The Hpo pathway was originally identified in Drosophila as a potent regulator of inhibition of cell growth and promotion of apoptosis. The pathway consists of a tumor suppressor kinase cascade which negatively regulates growth and results in inactivation of a transcriptional co-activator, Yorkie (Yki)[8]. The human ortholog of Yki, YAP, has a 31% sequence homology and similar biologic activity. YAP is a 65 kDa phosphoprotein which is rich in proline. In biological conditions, YAP is phosphorylated by the Hpo signaling pathway, and is highly conserved with other components of this pathway, regulating the balance between cell proliferation and apoptosis to maintain the steady-state of the cellular environment[2910]. Dysregulation of any factor(s) in this pathway can lead to tumorigenesis. Overholtzer et al[3] introduced the YAP gene by retroviral infection into the immortalized, but non-tumorigenic, human mammary epithelial cell line MCF10A and found that overexpression of YAP induced epithelial-to-mesenchymal transition, suppression of apoptosis, growth factor-independent proliferation, and anchorage-independent growth in soft agar, which suggests that YAP contributes to malignant transformation in cancers, and supports the potential significance of this pathway in human cancer.
Zhao et al[2] evaluated YAP expression in 115 cases of human hepatocellular carcinoma (HCC) samples by IHC staining of tissue microarrays. Among the 115 cases of human HCC samples examined, 54% showed strong YAP staining, while 95% of normal liver tissue samples (40 out of 42 cases) showed very weak staining, indicating a significant difference in YAP levels between normal and cancerous tissues. Similar observations were made in prostate cancer tissues. Up to now, we have found only one report on the expression and role of YAP expression in gastric carcinoma, where YAP expression in 78 normal gastric mucosa was 14%, while in 55 gastric carcinomas and 92 gastric metastatic disease the expression was 30% and 35% respectively, significantly higher than that in normal gastric mucosa[11]. Our IHC investigation found that the expression of YAP in DYS and gastric carcinoma was significantly higher than in normal gastric mucosa, suggesting that an abnormality of the Hpo pathway leads to overexpression of YAP, resulting in malignant transformation of the gastric mucosa. We speculate that YAP may play an important role as a tumorigenic factor and early gastric tumorigenic molecule during gastric carcinogenesis.
Survivin is a new member of the IAP family, and has been implicated to have a role in protection from apoptosis and regulation of mitosis[12]. The survivin gene has been located on the 17q25 chromosome, encoding a 16.5 kDa protein. Survivin is characterized by a unique structure with a single BIR on the N terminal and an α-helix structure on the C terminal; the BIR structure is thought to play a role during anti-apoptosis, while the helix structure may participate in the microtubule binding structure[1314]. Data from a large analysis of human transcripts revealed survivin as the fourth most highly expressed protein in human cancer tissue compared with normal tissue[15–18]. Xiao et al[19] found that the positive rates of survivin expression in tumors with metastases (in lymph node metastasis 86.2%, liver metastasis 100% and ovarian metastasis 100%) were significantly higher than that in tumors without metastasis (64.3%). Our data indicated that the positive rates of survivin in IM, atypical hyperplasia and gastric carcinoma were significantly higher than that in normal gastric mucosa. The expression level gradually increased from well differentiated adenocarcinoma, through moderately differentiated adenocarcinoma to poorly differentiated adenocarcinoma, with significant Rank correlation. The positive rate of survivin in gastric carcinoma of the diffused type was significantly higher than that in the intestinal type. In gastric carcinoma with lymph node metastasis, the positive rate of survivin was significantly higher than that in the group without lymph node metastasis, indicating that survivin may be involved in the occurrence, development and lymph node metastasis of gastric carcinoma. Survivin can act as a prognostic and predictive indicator for gastric carcinoma patients.
Dong et al[7] used microarray analysis to identify YAP-induced genes in murine livers. Selected genes from the microarray analysis were validated by real-time quantitative polymerase chain reaction analysis. YAP induced the transcription of many genes which are normally associated with hepatocyte proliferation, such as Ki67, c-myc, SOX4, H19, and AFP. It also induced the expression of several negative regulators of apoptosis, such as the IAP family members BIRC5/survivin and BIRC2/cIAP1, and the BCL2 family gene MCL1. Of note is the massive induction (30-fold) of BIRC5/survivin. To determine whether cIAP1 and YAP might cooperate during tumorigenesis, p53-/-, myc liver progenitor cells were infected with either YAP and control vector or YAP plus cIAP1 and assayed for their ability to form tumors in vivo. Tumors arising from p53-/-, myc hepatoblasts coexpressing cIAP1 and YAP grew faster than those expressing either oncogene alone, suggesting that they may collaborate to contribute to tumorigenesis and progression[20].
Our investigation found that the expression of YAP and survivin in gastric carcinoma were positively correlated, and we speculate that YAP may induce a high expression of cell proliferation-related factors and apoptotic inhibitors, such as Ki67, cIAP1 and survivin. Survivin may participate in gastric carcinogenesis, progression and metastasis by inhibiting apoptosis of gastric carcinoma and regulating cellular mitosis. Whether YAP and survivin collaborate to contribute to gastric carcinogenesis and progression require further study.
Previous reports have found that YAP was an activator of cell death in mammalian cells. YAP was shown to activate apoptosis in response to DNA damage by interacting with p73 in several cancer cell lines[2122]. This is in direct contrast to the results of our investigation and other previous reports. The roles of YAP in biological and pathological conditions remain to be clearly defined.
Yes-associated protein (YAP) is a type of cellular adaptor protein and transcriptional co-activator. In recent years, some investigators have found YAP to be overexpressed and highly activated in hepatic cancers and mammary cancers, suggesting its tumorigenicity. Survivin is a new member of the inhibitor of apoptotic protein (IAP) family, which was initially cloned by the cDNA of the effector cell protease receptor-1 in the human genomic library in 1997. The authors measured the expression of YAP and survivin in normal gastric mucosa, precancerous lesions and gastric carcinoma using an immunohistochemical method, to analyze the significance and correlations of the two factors with gastric carcinogenesis.
The Hippo (Hpo) pathway was originally identified in Drosophila as a potent regulator of inhibition of cell growth and promotion of apoptosis. The pathway consists of a tumor suppressor kinase cascade which negatively regulates growth and results in inactivation of a transcriptional co-activator, Yorkie (Yki). The human ortholog of Yki, YAP, has a 31% sequence homology and similar biologic activity. YAP is a 65 kDa phosphoprotein, rich in proline. In biological conditions, YAP is phosphorylated by the Hpo signaling pathway, and is highly conserved with other components of this pathway, regulating the balance between cell proliferation and apoptosis to maintain the steady-state of the cellular environment.
Previous reports have found that YAP was an activator of cell death in mammalian cells. YAP was shown to activate apoptosis in response to DNA damage by interacting with p73 in several cancer cell lines. This is in direct contrast to the results of their investigation and other previous reports.
The authors investigation found that the expression of YAP and survivin in gastric carcinoma were positively correlated. They speculate that YAP may induce a high expression of cell proliferation-related factors and apoptotic inhibitors, such as Ki67, cIAP1 and survivin. Detecting YAP and survivin together may help in early diagnosis of gastric carcinoma. Whether YAP and survivin collaborate to contribute to gastric tumorigenesis and progression requires further study.
The investigation found that the expression of YAP and survivin in gastric carcinoma were positively correlated, and the authors speculated that YAP may induce a high expression of cell proliferation-related factors and apoptotic inhibitors, such as Ki67, cIAP1 and survivin. Survivin may participate in gastric carcinogenesis, progression and metastasis by inhibiting apoptosis of gastric carcinoma cells and regulating cellular mitosis. The study is interesting and is worth further exploration.
| 1. | Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145-2152. |
| 2. | Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747-2761. |
| 3. | Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405-12410. |
| 4. | Jiang Q, Liu D, Gong Y, Wang Y, Sun S, Gui Y, Song H. yap is required for the development of brain, eyes, and neural crest in zebrafish. Biochem Biophys Res Commun. 2009;384:114-119. |
| 5. | Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-921. |
| 6. | Lehner R, Enomoto T, McGregor JA, Shroyer L, Haugen BR, Pugazhenthi U, Shroyer KR. Correlation of survivin mRNA detection with histologic diagnosis in normal endometrium and endometrial carcinoma. Acta Obstet Gynecol Scand. 2002;81:162-167. |
| 7. | Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120-1133. |
| 8. | Wang K, Degerny C, Xu M, Yang XJ. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009;87:77-91. |
| 10. | Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675-685. |
| 11. | Lam-Himlin DM, Daniels JA, Gayyed MF, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. The hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway. Int J Gastrointest Cancer. 2006;37:103-109. |
| 12. | Sun C, Nettesheim D, Liu Z, Olejniczak ET. Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry. 2005;44:11-17. |
| 13. | Zhang M, Mukherjee N, Bermudez RS, Latham DE, Delaney MA, Zietman AL, Shipley WU, Chakravarti A. Adenovirus-mediated inhibition of survivin expression sensitizes human prostate cancer cells to paclitaxel in vitro and in vivo. Prostate. 2005;64:293-302. |
| 14. | Blum R, Jacob-Hirsch J, Rechavi G, Kloog Y. Suppression of survivin expression in glioblastoma cells by the Ras inhibitor farnesylthiosalicylic acid promotes caspase-dependent apoptosis. Mol Cancer Ther. 2006;5:2337-2347. |
| 15. | Rodríguez JA, Span SW, Ferreira CG, Kruyt FA, Giaccone G. CRM1-mediated nuclear export determines the cytoplasmic localization of the antiapoptotic protein Survivin. Exp Cell Res. 2002;275:44-53. |
| 16. | Tarnawski A, Pai R, Chiou SK, Chai J, Chu EC. Rebamipide inhibits gastric cancer growth by targeting survivin and Aurora-B. Biochem Biophys Res Commun. 2005;334:207-212. |
| 17. | Rosa J, Canovas P, Islam A, Altieri DC, Doxsey SJ. Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Mol Biol Cell. 2006;17:1483-1493. |
| 18. | Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, Lal A, Wang CJ, Beaudry GA, Ciriello KM. Analysis of human transcriptomes. Nat Genet. 1999;23:387-388. |
| 19. | Xiao YP, Lin Z, Mao LL, Wu DY, Gao YJ, Sun HW, Xin Y. Significance and expression of Bax, Survivin and p53 in gastric carcinoma and precancerous lesions using tissue microarray. Chinese-German J Clin Oncol. 2007;6:302-304. |
| 20. | Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253-1267. |