Published online Aug 14, 2009. doi: 10.3748/wjg.15.3819
Revised: July 17, 2009
Accepted: July 24, 2009
Published online: August 14, 2009
We present a 69-year-old woman with a duodenal obstruction after successful selective transcatheter arterial embolization (TAE) for a duodenal diverticular hemorrhage. Two weeks after TAE, the patient showed abrupt symptoms of duodenal obstruction. Resolving hematomas after successful selective transcatheter arterial embolization should be thoroughly observed because they might result in duodenal fibrotic encasement featuring inflammatory duodenal wall thickening, duodenal deformity, dysmotility, and finally obstruction.
- Citation: Kwon YJ, Kim JH, Kim SH, Kim BS, Kim HU, Choi EK, Jeong IH. Duodenal obstruction after successful embolization for duodenal diverticular hemorrhage: A case report. World J Gastroenterol 2009; 15(30): 3819-3822
- URL: https://www.wjgnet.com/1007-9327/full/v15/i30/3819.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3819
Duodenal diverticulum is a disease entity that was first described in 1710 by Chromel. Most duodenal diverticula are diagnosed incidentally, 2% to 5% at barium study of gastrointestinal tract, 12% to 27% at endoscopy, and 22% in autopsy cases. Only 5% to 10% of patients with duodenal diverticula suffer from clinical symptoms. However, less than 1% of patients require treatment for various complications such as perforation, hemorrhage, and biliary/pancreatic/gastrointestinal obstruction.
Here, we report a patient who presented with duodenal obstruction after successful selective transcatheter arterial embolization (TAE) for a duodenal diverticular hemorrhage.
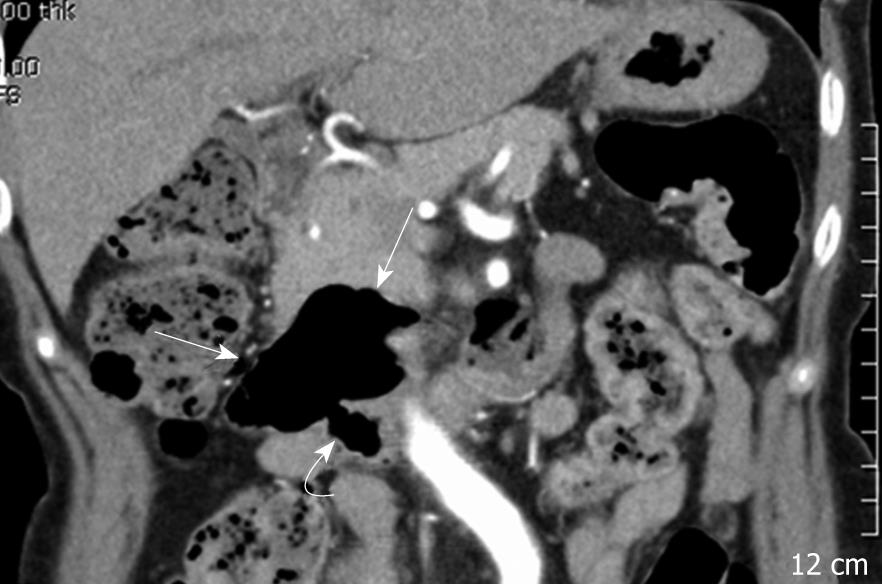
A 69-year-old woman was admitted with abrupt onset of dull abdominal pain and vomiting. She had hypertension, did not drink alcohol, smoke, or use non-steroidal anti-inflammatory drugs. She had visited the department of internal medicine complaining principally of melena two years prior to admission. At that time, initial serum hemoglobin was 12.2 g/dL, and endoscopic evaluation, including gastroscopy and colonoscopy, showed no specific findings except for erosive gastritis. Computed tomography (CT) images showed a large duodenal diverticulum, however it was decided to merely observe the diverticulum because it did not cause any symptoms (Figure 1).
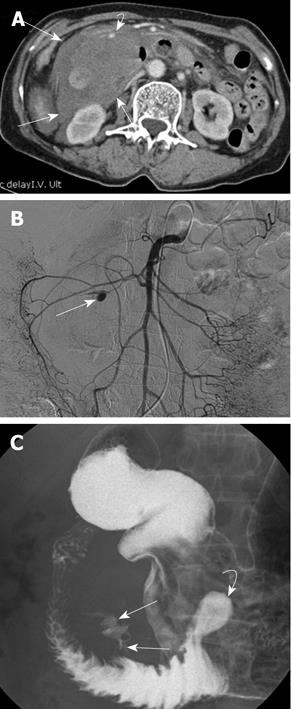
When she arrived at the emergency room this time, an intraabdominal bulging mass was palpated in the right upper quadrant of her abdomen. Her hemoglobin level was 7.9 g/dL and a CT scan showed an acute duodenal diverticular hemorrhage, large surrounding retroperitoneal hematoma, and an arterial pseudoaneurysm. The arterial pseudoaneurysm was thought to be the cause of the acute hemorrhage (Figure 2A). A superior mesenteric arteriogram (Figure 2B), showed that the arterial pseudoaneurysm was in a duodenal branch of the anteroinferior pancreaticoduodenal artery and transcatheter glue embolization was performed in a superselective way using a Histoacryl (N-butyl-2-cyanoacrylate)-Lipiodol mixture. A post-embolization arteriogram showed successful embolization of the pseudoaneurysm.
Gastroscopy and an upper gastrointestinal barium study performed on the seventh day after the embolization revealed a huge diverticulum at the mesenteric border of the duodenal third portion, about 10 cm distal from the ampulla. The duodenal lumen was compressed by the duodenal diverticulum and surrounding hematoma. Another small diverticulum was identified at about 10 cm distal to the complicated diverticulum. There was no evidence of further active bleeding from the upper gastrointestinal tract or any ischemic changes to the duodenum. The duodenal passage was intact (Figure 2C).
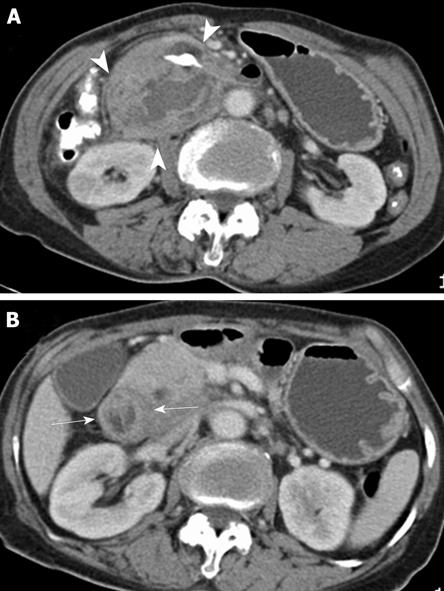
Two weeks after admission, the patient showed gastrointestinal symptoms of obstruction (epigastric distension and postprandial bilious vomiting). A follow-up CT scan showed a decrease in the duodenal diverticular and the retroperitoneal hematoma; however, marked edematous duodenal wall thickening and luminal narrowing had developed in the duodenal second and third portions, suggesting duodenal deformity resulting from peridiverticular fibrosis (Figure 3). Despite the conservative treatment with total parenteral nutrition (TPN) and Levin tube drainage, the patient’s symptoms of obstruction did not improve. Furthermore, TPN was no longer possible because of repeated central line sepsis. Therefore, a laparotomy was performed 24 d after the admission.
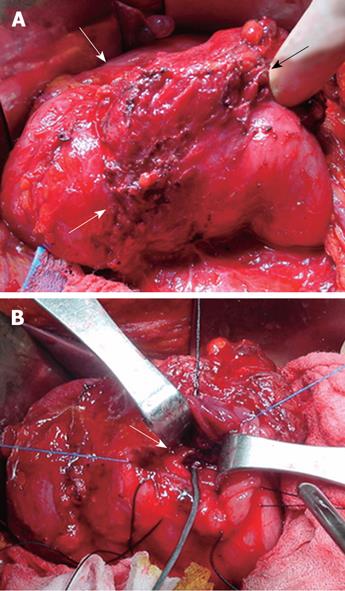
During the laparotomy, we found a hard duodenal diverticulum filled with a blood clot, which covered about 10 cm of the duodenal second portion. In addition, severe peridiverticular fibrosis and deformity were found to be causing duodenal second portion stricture. Through a longitudinal duodenotomy, a diverticular opening was identified at the 3rd portion of the duodenum that was more than 10 cm away from the ampulla of Vater. Neither dissection nor resection of the diverticulum was possible due to dense adhesion. Instead, suture ligation of the diverticular opening, with hematoma evacuation and gastrojejunostomy, was performed (Figure 4).
After the operation, she suffered from delayed gastric emptying, which caused symptoms such as postprandial bilious vomiting. However, the symptoms improved dramatically after several weeks of conservative treatment and, open discharge, she retained the ability to tolerate any meal without bleeding.
We have presented a patient with duodenal obstruction after successful selective TAE for a duodenal diverticular hemorrhage. There are few reports on duodenal obstruction caused by duodenal stenosis from retroperitoneal or duodenal fibrotic encasement, while a few other reports describe the obstruction caused by intraluminal diverticular stone, bezoar, and ingested foreign bodies around diverticulum[1–3]. To the best of our knowledge, this is the first case report of duodenal diverticular hemorrhage complicated by duodenal obstruction after TAE.
Only 5% to 10% of patients with duodenal diverticula suffer from clinical symptoms, of which 1% or less require treatment for various complications such as perforation, hemorrhage, and biliary/pancreatic/gastrointestinal obstruction[14]. In our patient, the patient suffered from both hemorrhage and obstruction. After a CT scan and from laboratory findings, we found that the symptoms of obstruction were caused by the diverticular hemorrhage and a surrounding retroperitoneal hematoma, which compressed the duodenal lumen. Angiography showed that the origin of the hematoma was the arterial pseudoaneurysmal rupture on the anterior wall of the duodenal diverticulum.
Use of arteriography to diagnose duodenal diverticular bleeding was first reported by Miller et al[5] in 1970. Since then, angiography and endoscopy have become the most useful modalities for diagnosing and managing gastrointestinal bleeding, suggesting TAE as the treatment of choice of duodenal diverticular hemorrhage[67]. However, due to the complex vascular anatomy of duodenum, TAE must be done with extreme caution. Pancreatitis, ischemic damage, and rebleeding have been reported as possible complications after superselective TAE. In our patient, the patient didn’t suffer from any of these complications after the superselective TAE, and bleeding control was successful with no change in hemoglobin level during the follow-up period after the intervention. However, two weeks after admission, symptoms of obstruction were abruptly aggravated. CT, an upper gastrointestinal barium study, and gastroscopy suggested stricture of the second portion of the duodenum caused by duodenal wall thickening with deformity and dysmotility, which resulted from peridiverticular fibrosis, not by ischemic damage from the embolization. Therefore, great care should be taken after the TAE, not only to detect pancreatitis and rebleeding, but also for the symptoms of obstruction, so that the patient can be managed appropriately and timely.
According to previous reports, duodenal obstruction due to fibrotic encasement of the duodenum in most cases is self-limited by the end of the first month of treatment of nasogastric suction and total parenteral nutrition[8]. However, the patient suffered from repeated central line sepsis, therefore we concluded that the conservative treatment had failed and we decided to operate.
Various operations are considered for diverticulum based on the symptoms and operative field findings[1910]. Surgical management of duodenal obstruction in duodenal diverticulum includes diverticulectomy through duodenotomy or duodenal resection with primary end-to-end anastomosis. However, inflamed duodenal diverticulum might cause adhesions, making it difficult to dissect, especially when the diverticulum is located in the second portion of the duodenum. The diversion of the upper gastrointestinal tract might be better than complete dissection and resection of the diverticulum, possibly preventing CBD or AoV injury. Therefore, a diversion procedure is more suggestive when there is severe inflammation or friability of the tissue. In our patient, the diverticular opening was in the 3rd portion of the duodenum; however, due to severe dense adhesions and fibrosis of the duodenum, only suture ligation of diverticular opening and gastrojejunostomy were performed.
In conclusion, selective TAE should be considered as the first line treatment for duodenal diverticular hemorrhage. Complications of peridiverticular and retroperitoneal fibrosis around the resolving hematoma could happen after successful TAE; therefore the resolving hematoma should be thoroughly observed. In addition, conservative treatment should be considered before surgery to relieve the duodenal obstruction resulting from duodenal fibrotic encasement after a duodenal diverticular hemorrhage. If surgery is necessary, gastrointestinal diversion could be done instead of complete resection in cases with severe inflammation or tissue friability.
| 1. | Mathis KL, Farley DR. Operative management of symptomatic duodenal diverticula. Am J Surg. 2007;193:305-308; discussion 308-309. |
| 2. | Ferri L, Feldman L. Obstructing duodenal diverticula. J Am Coll Surg. 2002;195:888-889. |
| 3. | Harthun NL, Morse JH, Shaffer HA Jr, Minasi JS. Duodenal obstruction caused by intraluminal duodenal diverticulum and annular pancreas in an adult. Gastrointest Endosc. 2002;55:940-943. |
| 4. | Martinez-Cecilia D, Arjona-Sanchez A, Gomez-Alvarez M, Torres-Tordera E, Luque-Molina A, Valenti-Azcarate V, Briceno-Delgado J, Padillo FJ, Lopez-Cillero P, Rufian-Pena S. Conservative management of perforated duodenal diverticulum: a case report and review of the literature. World J Gastroenterol. 2008;14:1949-1951. |
| 5. | Miller RE, McCabe RE, Salomon PF, Knox WG. Surgical complications of small bowel diverticula exclusive of Meckel's. Ann Surg. 1970;171:202-210. |
| 6. | Yin WY, Chen HT, Huang SM, Lin HH, Chang TM. Clinical analysis and literature review of massive duodenal diverticular bleeding. World J Surg. 2001;25:848-855. |
| 7. | Poultsides GA, Kim CJ, Orlando R 3rd, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008;143:457-461. |
| 8. | Touloukian RJ. Protocol for the nonoperative treatment of obstructing intramural duodenal hematoma during childhood. Am J Surg. 1983;145:330-334. |
| 9. | Zoepf T, Zoepf DS, Arnold JC, Benz C, Riemann JF. The relationship between juxtapapillary duodenal diverticula and disorders of the biliopancreatic system: analysis of 350 patients. Gastrointest Endosc. 2001;54:56-61. |