Published online Aug 14, 2009. doi: 10.3748/wjg.15.3783
Revised: July 6, 2009
Accepted: July 14, 2009
Published online: August 14, 2009
AIM: To elucidate the efficacy and safety of a split dose of midazolam in combination with meperidine for colonoscopy.
METHODS: Eighty subjects undergoing outpatient colonoscopy were randomly assigned to group A or B. Group A (n = 40) received a split dose of midazolam in combination with meperidine. Group B (n = 40) received a single dose of midazolam in combination with meperidine. Outcome measurements were level of sedation, duration of sedation and recovery, degree of pain and satisfaction, procedure-related memory, controllability, and adverse events.
RESULTS: Group A had a lower frequency of significant hypoxemia (P = 0.043) and a higher sedation score on withdrawal of the endoscope from the descending colon than group B (P = 0.043). Group B recovered from sedation slightly sooner than group A (P < 0.002). Scores for pain and memory, except insertion-related memory, were lower in group A one week after colonoscopic examination (P = 0.018 and P < 0.030, respectively). Poor patient controllability was noted by the endoscopist and nurse in group B (P = 0.038 and P = 0.032, respectively).
CONCLUSION: Split dose midazolam in combination with meperidine resulted in a safer, more equable sedation status during colonoscopic examination and a reduction in procedure-related pain and memory, but resulted in longer recovery time.
- Citation: Lee H, Kim JH. Superiority of split dose midazolam as conscious sedation for outpatient colonoscopy. World J Gastroenterol 2009; 15(30): 3783-3787
- URL: https://www.wjgnet.com/1007-9327/full/v15/i30/3783.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3783
Colonoscopy is one of the most commonly performed medical procedures worldwide. Although recent advances in endoscopy have improved endoscope imagery and flexibility, colonoscopy remains a difficult and lengthy endoscopic procedure that causes the patient considerable discomfort or pain. Most gastroenterologists use moderate sedation for colonoscopy. Under deep sedation, patients may develop inadequate spontaneous ventilation and may require assistance to maintain a patent airway. In addition, constant repositioning of such patients because of their poor cooperation may exhaust nurses or assistants[1–7].
Although several agents are available for inducing moderate sedation, the combination of a benzodiazepine and an opiate (midazolam and meperidine) is most commonly used[37–9]. The use of propofol is increasing in clinical practices because practitioners believe that it shortens the duration of sedation and recovery[10–15]. However, many endoscopic units, especially in Korea, are hesitant to use this agent because it frequently results in deep sedation. Moreover, it is doubtful whether it is suitable for colonoscopic procedures because of poor patient control[2416]. A modified administration of a conventional combination of sedatives such as midazolam and meperidine may be safer and have a more positive outcome than propofol, especially for colonoscopic procedures, the duration of which is greater and more variable than that of upper gastrointestinal endoscopy.
The objective of this study was to compare the efficacy and safety of split dose midazolam in combination with meperidine with conventional single dose midazolam in combination with meperidine.
This study was a randomized, controlled clinical trial and involved 80 consecutive outpatients who presented for colonoscopy at the Armed Forces Capital Hospital in Korea. Patients were considered eligible to participate if they were 18 years of age or older and were scheduled for colonoscopy only. Associated medical illnesses were graded according to the American Society of Anesthesiologists’ Physical Status Classification (ASA grade). Exclusion criteria included ASA risk Class 3 or higher, history of a colonic surgical procedure, inpatient status, chronic use of benzodiazepines or opiates, sleep apnea, liver cirrhosis, pregnancy, willingness to undergo unsedated colonoscopy, and allergies to soybeans or eggs. A total of 86 patients were invited to participate. Six of these patients were excluded because the colonoscopy was aborted due to poor preparation. Thus, 80 patients gave their informed consent and completed the study. All colonoscopies were performed by one gastroenterologist who had 6 years of colonoscopy experience. The sedative agents were administered by one nurse. The study was blinded to the endoscopist and recovery nurse, but not to the sedating nurse. Sedation endpoints were drowsiness, facial relaxation, slurred speech, and tolerance to insertion of the colonoscope. Patients were randomly assigned to one of the two groups for sedation according to a table of random numbers. All patients received 50 mg of meperidine intravenously prior to administration of midazolam. Patients in group A (split dose midazolam and meperidine) initially received 2.5 mg (< 70 kg b.w.) or 3 mg (> 70 kg b.w.) of midazolam intravenously. A second dose of midazolam (< 70 kg b.w., 1.5 mg; > 70 kg b.w., 2 mg) was administered immediately after ileal intubation. Patients in group B (single dose midazolam and meperidine) initially received 4 mg (< 70 kg b.w.) or 5 mg (> 70 kg b.w.) of midazolam intravenously. Placebo administration of saline was performed after ileal intubation in group B. Supplemental doses of midazolam or meperidine were not administered to ensure that consistent doses were used during the trial. The times at which sedation and colonoscopic insertion were initiated, the distal ileum was intubated, and the colonoscope was withdrawn from the anus were recorded.
The endoscopist and registered nurse who administered the sedative agent were certified in advanced cardiac life support. An endoscopy technician was present to assist the endoscopist with technical maneuvers. All patients were continuously monitored for heart rate (three-lead electrocardiogram), oxygen saturation (pulse oximetry), and mean arterial blood pressure (serial blood pressure measurements every 5 min). Significant oxygen desaturation was defined as an oxygen saturation of less than 90% for more than 15 s. Supplemental oxygen (2 L/min via a nasal cannula) was administered for any episode in which SaO2 was less than 85% or oxygen saturation was less than 90% on three separate occasions. Oxygen desaturation and the need for supplemental oxygen were recorded as significant outcomes. Heart rate was monitored continuously using pulse oximetry and bradycardia was defined as a heart rate of less than 60 beats per minute or a heart rate 25% below baseline. Blood pressure was measured before and after the procedure.
After sedation was initiated, the assistant responsible for measuring sedation level was brought into the room. The assistant was unaware of the protocol concerning delivery of the sedative. The degree of sedation was assessed using a sedation score (5 = not arousable, 4 = arousable to stimuli, 3 = arousable to command, 2 = drowsy, 1 = awake). The sedation score was recorded twice for each patient: on advancing the endoscope into the descending colon and on withdrawing it from the descending colon. Patients were transferred to the recovery area immediately after the procedure if their vital signs were stable. The time taken to recover from sedation was assessed using a modified Aldrete score at 5, 10, and 30 min after the procedure. The modified Aldrete score is an established postanesthetic recovery score that takes into account respiration and circulation parameters, and patient consciousness and activity[17]. The score ranges from 0 to 10, the latter indicating that the patient is fit for discharge.
When the patients were contacted by phone or visited one week after the procedure, they were asked to fill out a questionnaire using a 10-point visual analog scale to grade the amount of pain, overall satisfaction, and memory of specific aspects of the procedure (insertion of the colonoscope, withdrawal of the colonoscope, and intentional position changes on ileal intubation). This was conducted by an investigator blinded to the protocol of study.
The endoscopist’s and assistant nurse’s evaluations of the patient’s controllability were obtained immediately after the procedure using a 10-point visual analog scale.
On the basis of a previous study[18], assuming that the split dose group will have a mean procedure-related pain score of 8.0/10.0 with a 1% reduction in pain, a sample size of 37 per group was required to detect the difference with a power of 90% and a type I error of 0.05 (PS Power and Sample Size Calculations, Version 3.0; Biostatistics Department, Vanderbilt University, Nashville, TN). Descriptive data are presented as the number of patients (%) or as the mean ± SD. Continuous data were compared using the unpaired Student’s t test, and categorical variables were tested using the corrected chi-square method. The criterion for statistical significance was P < 0.05.
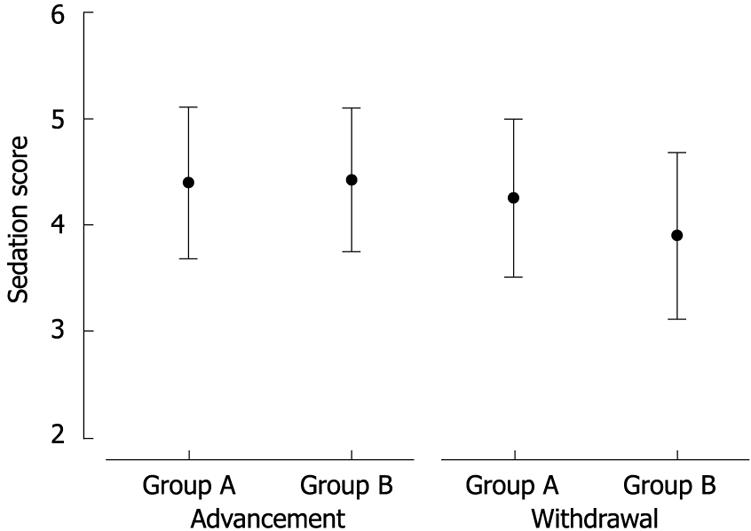
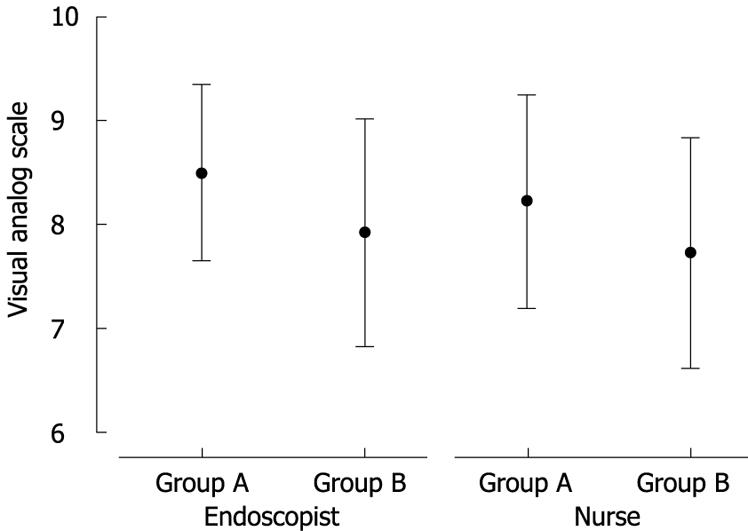
Demographic data and procedure indications for the subjects are presented in Table 1. There were no significant differences among the groups in age, gender, body mass index, or procedure indication. Patients who received single dose midazolam had a significantly shorter time from onset of sedation to initiation of endoscopy than those who received split dose midazolam (P = 0.003, 0.89 ± 0.31 vs 1.18 ± 0.49). There was no difference in the total duration of the procedure and the time of ileal intubation between patients in both groups. The frequency of a significant drop in oxygen saturation was higher in group B than in group A (P = 0.043, 20% vs 5%). There was no difference between the groups in terms of a significant drop in mean arterial blood pressure or a significant alteration in heart rate (Table 2). Reversal agents were needed in two cases. However, no patient experienced severe cardiopulmonary complications. There was a significant difference between the sedation scores in group A and B at the time at which the scope was advanced into the descending colon. However, the sedation score in group A was significantly higher than that in group B on withdrawal of the endoscope from the descending colon (4.25 ± 0.74 vs 3.9 ± 0.78, P = 0.043) (Figure 1). Table 3 shows recovery status according to the modified Aldrete score. Recovery from sedation was slightly sooner for group B than for group A, 5 min (4.28 ± 1.06 vs 5.75 ± 0.95, respectively, P < 0.001), 10 min (6.55 ± 0.50 vs 7.00 ± 0.68, respectively, P < 0.001), and 30 min (8.73 ± 0.85 vs 9.32 ± 0.69, respectively, P < 0.001) after the procedure. Moreover, the interval from removal of the endoscope to discharge was longer for group A than group B (37.1 ± 6.87 min vs 33.8 ± 5.75 min, respectively, P = 0.022). The patients’ recollection of pain during colonoscopy and satisfaction with the procedure at one week after the procedure are shown in Table 4. One week after the colonoscopic examination, all scores for pain and memory except for insertion-related memory were lower for group A than for group B. The endoscopist and the assistant nurse reported that group B was more difficult to control during the procedure than group A (P = 0.038 and P = 0.032, respectively) (Figure 2).
| Group A (n = 40) | Group B (n = 40) | P value | |
| Age (yr) | 31.33 ± 9.76 | 31.23 ± 9.13 | 0.962 |
| Gender (M/F) | 34/6 | 36/4 | 0.737 |
| Body mass index | 23.80 ± 3.40 | 23.43 ± 3.78 | 0.642 |
| No. of patients with previous colonoscopy (%) | 11 (27.5%) | 10 (25.0%) | 0.779 |
| Indication for colonoscopy n (%) | |||
| Change in bowel habit | 9 (22.5) | 10 (25.0) | 0.527 |
| Abdominal pain | 10 (25.0) | 12 (30.0) | |
| Rectal bleeding | 6 (15.0) | 7 (17.5) | |
| Polyp surveillance | 13 (32.5) | 9 (22.5) | |
| Anemia | 2 (5.0) | 2 (5.0) | |
| Time from onset of sedation to scope (min) | 1.18 ± 0.50 | 0.89 ± 0.31 | 0.003 |
| Total procedure time (min) | 14.80 ± 3.80 | 14.61 ± 3.83 | 0.824 |
| Time to ileal intubation (min) | 7.49 ± 3.24 | 8.21 ± 3.37 | 0.328 |
| Group A (n = 40) | Group B (n = 40) | P value | |
| Baseline systolic BP (mmHg) | 125.20 ± 9.76 | 125.40 ± 10.15 | 0.929 |
| Baseline pulse rate (/min) | 71.90 ± 9.71 | 72.63 ± 8.99 | 0.730 |
| Baseline oxygen saturation (%) | 97.88 ± 1.98 | 97.95 ± 2.04 | 0.868 |
| Drop in blood pressure | 1 (2.5%) | 2 (5.0%) | 0.556 |
| Alteration in heart rate | 3 (7.5%) | 2 (5.0%) | 0.745 |
| Drop in oxygen saturation | 2 (5.0%) | 8 (20.0%) | 0.043 |
| Group A (n = 40) | Group B (n = 40) | P value | |
| Aldrete score (5 min) | 4.28 ± 1.06 | 5.75 ± 0.95 | < 0.001 |
| Aldrete score (10 min) | 6.55 ± 0.50 | 7.00 ± 0.68 | 0.001 |
| Aldrete score (30 min) | 8.73 ± 0.85 | 9.32 ± 0.69 | 0.001 |
| Time from scope out to discharge | 37.05 ± 6.87 | 33.75 ± 5.75 | 0.022 |
| Group A (n = 40) | Group B (n = 40) | Pvalue | |
| Pain | 0.95 ± 1.15 | 1.58 ± 1.15 | 0.018 |
| Memory | |||
| On insertion | 0.80 ± 0.82 | 1.08 ± 0.89 | 0.155 |
| On ileal intubation | 1.38 ± 1.21 | 1.93 ± 0.94 | 0.026 |
| On scope out | 0.93 ± 1.02 | 1.58 ± 1.38 | 0.019 |
| Satisfaction | 8.93 ± 1.07 | 8.68 ± 1.09 | 0.305 |
This study is the first to compare the safety and efficacy of split dose midazolam in combination with meperidine with that of single dose midazolam in combination with meperidine for conscious sedation during colonoscopy. Although propofol is used increasingly during routine colonoscopy, nonanesthesiologists are hesitant to use propofol because of its known potential to cause transient apnea or general anesthesia, for which there is no reversal agent[2419].
There is a widespread belief among endoscopists that, for colonoscopy, sedation with the combination of a benzodiazepine and a narcotic is superior to sedation with either agent alone. A recent uncontrolled prospective study of a cohort of patients sedated using a combination of midazolam and meperidine reported that unintended deep sedation occurred in 11% of colonoscopies[11]. Our aim was to identify a practical and alternative sedation method based on the combination of a benzodiazepine and meperidine. Most endoscopists prescribe single dose midazolam at the time of initiating the colonoscopy without adequately monitoring the patient’s sedative status or the amount of additional sedative administered. Endoscopists routinely prescribe additional administration of a sedative when patients are severely agitated or experience pain, but there are no coherent guidelines for this practice. Moreover, the small painful response of patients during the procedure tends to be neglected by endoscopic unit staff. This randomized study was designed to evaluate the promising effect of a divided dose of midazolam on sedation status.
Midazolam is known to produce retrograde amnesia as well as anterograde amnesia[20]. Because of the long duration of colonoscopic procedures, it is thought that single dose midazolam administration results in uneven distribution of the sedative effect during the procedure. Moreover, administration of a single full dose of midazolam is associated with the risk of hypoxemia and paradoxical reactions such as hostility, rage, and physical violence, especially during the initial part of the procedure. For these reasons, an alternative method is required to ensure adequate moderate sedation for the duration of colonoscopy. Our study showed that the sedative efficacy of the split dose was superior to that of the single dose at the time of withdrawal of the endoscope. Although there was no serious cardiopulmonary depression in either group, the split dose reduced the frequency of a drop in oxygen saturation, unlike the single dose. Split dose midazolam treatment is a stable, sustainable, and safe sedation method for colonoscopy. Unfortunately, the post endoscopic sedation-related recovery of the split dose group was delayed according to the Aldrete score. It seems inevitable that additional administration of sedative will affect the recovery of patients.
The split dose method induced sleep by the end of the colonoscopic procedure and was associated with loss of memory of the events that occurred during colonoscopy 1 wk later. It seems that split dose midazolam administration and the subsequent post endoscopic sleep played a role in the amnesia. This should be considered for moderate sedation even if the main concern is to facilitate rapid recovery to ensure fast turnover at the endoscopic unit.
Initial excessive sedation to ensure easy patient control during colonoscopy seems to be undesirable at the time of advancement of the endoscope. Our study suggests that less sedation should be administered during advancement of the endoscope and more sedation with the moderate agent should be administered during endoscope withdrawal. Regrettably, the satisfaction score in our study was not different between the groups. This seems to have been caused by an inadequate number of subjects because the sampling size was calculated on the basis of pain score in a previous report. Hence, further study with a larger number of subjects should be considered.
In summary, our study shows that a split dose of midazolam in combination with meperidine is superior to a single dose of midazolam in combination with meperidine with respect to safety, equable sedation status, procedure-related pain, and unpleasant memory, but results in a longer recovery time.
For conscious sedation during colonoscopy, the modified administration of a conventional sedative combination such as midazolam and meperidine may be safer and have a more positive outcome than propofol.
Researchers assessed the efficacy of various premedications such as propofol, midazolam, meperidine or fentanyl on moderate sedation during the colonoscopic procedure.
Split dose midazolam in combination with meperidine led to a lower frequency of significant hypoxemia and a higher sedation score on withdrawal of the colonoscope. One week after colonoscopic examination, scores for pain and memory were lower in patients who received split dose midazolam compared to those who received single dose midazolam.
A split dose of midazolam in combination with meperidine is superior to a single dose of midazolam in combination with meperidine with respect to safety, equable sedation status, procedure-related pain, and unpleasant memory.
This is a randomized study to compare the safety and efficacy of split dose midazolam in combination with meperidine with that of single dose midazolam in combination with meperidine for conscious sedation during colonoscopy. This manuscript suggested that split dose midazolam treatment is a stable, sustainable, and safe sedation method for colonoscopy compared with the administration of single dose midazolam.
| 1. | Seeff LC, Richards TB, Shapiro JA, Nadel MR, Manninen DL, Given LS, Dong FB, Winges LD, McKenna MT. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670-1677. |
| 2. | Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017. |
| 3. | Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317-322. |
| 4. | Faigel DO, Baron TH, Goldstein JL, Hirota WK, Jacobson BC, Johanson JF, Leighton JA, Mallery JS, Peterson KA, Waring JP. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc. 2002;56:613-617. |
| 5. | Rex DK. Review article: moderate sedation for endoscopy: sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther. 2006;24:163-171. |
| 6. | Patel S, Vargo JJ, Khandwala F, Lopez R, Trolli P, Dumot JA, Conwell DL, Zuccaro G. Deep sedation occurs frequently during elective endoscopy with meperidine and midazolam. Am J Gastroenterol. 2005;100:2689-2695. |
| 7. | Froehlich F, Harris JK, Wietlisbach V, Burnand B, Vader JP, Gonvers JJ. Current sedation and monitoring practice for colonoscopy: an International Observational Study (EPAGE). Endoscopy. 2006;38:461-469. |
| 8. | Bell GD, Charlton JE. Colonoscopy--is sedation necessary and is there any role for intravenous propofol? Endoscopy. 2000;32:264-267. |
| 9. | Rex DK, Khalfan HK. Sedation and the technical performance of colonoscopy. Gastrointest Endosc Clin N Am. 2005;15:661-672. |
| 11. | McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67:910-923. |
| 12. | Cohen LB, Wecsler JS, Gaetano JN, Benson AA, Miller KM, Durkalski V, Aisenberg J. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967-974. |
| 13. | Faulx AL, Vela S, Das A, Cooper G, Sivak MV, Isenberg G, Chak A. The changing landscape of practice patterns regarding unsedated endoscopy and propofol use: a national Web survey. Gastrointest Endosc. 2005;62:9-15. |
| 14. | Tohda G, Higashi S, Wakahara S, Morikawa M, Sakumoto H, Kane T. Propofol sedation during endoscopic procedures: safe and effective administration by registered nurses supervised by endoscopists. Endoscopy. 2006;38:360-367. |
| 15. | Aisenberg J, Brill JV, Ladabaum U, Cohen LB. Sedation for gastrointestinal endoscopy: new practices, new economics. Am J Gastroenterol. 2005;100:996-1000. |
| 16. | Weston BR, Chadalawada V, Chalasani N, Kwo P, Overley CA, Symms M, Strahl E, Rex DK. Nurse-administered propofol versus midazolam and meperidine for upper endoscopy in cirrhotic patients. Am J Gastroenterol. 2003;98:2440-2447. |
| 17. | Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924-934. |
| 18. | Paspatis GA, Manolaraki M, Xirouchakis G, Papanikolaou N, Chlouverakis G, Gritzali A. Synergistic sedation with midazolam and propofol versus midazolam and pethidine in colonoscopies: a prospective, randomized study. Am J Gastroenterol. 2002;97:1963-1967. |
| 19. | Wehrmann T, Kokabpick S, Lembcke B, Caspary WF, Seifert H. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc. 1999;49:677-683. |