Published online Oct 28, 2008. doi: 10.3748/wjg.14.6188
Revised: July 17, 2008
Accepted: July 24, 2008
Published online: October 28, 2008
AIM: To investigate the effect of Gardenia jasminoides (GJ) on cerulein-induced acute pancreatitis (AP) in mice.
METHODS: C57BL/6 mice weighing 18-20 g were divided into three groups. (1) Normal saline-treated group, (2) treatment with GJ at a dose of 0.1 g/kg, (3) treatment with GJ at a dose of 1 g/kg. GJ was administered orally (n = 6 per group) for 1 wk. Three hours later, the mice were given an intraperitoneal injection of cerulein (50 μg/kg), a stable cholecystokinin (CCK) analogue, every hour for a total of 6 h as described previously. The mice were sacrificed at 6 h after completion of cerulein injections. Blood samples were obtained to determine serum amylase, lipase and cytokine levels. The pancreas was rapidly removed for morphologic examination and scoring. A portion of pancreas was stored at -70°C and prepared for the measurement of tissue myeloperoxidase (MPO) activity, an indicator of neutrophil sequestration, and for reverse-transcriptase PCR (RT-PCR) and real-time PCR measurements.
RESULTS: Treatment with GJ decreased significantly the severity of pancreatitis and pancreatitis-associated lung injury. Treatment with GJ attenuated the severity of AP compared with saline-treated mice, as shown by reduction in pancreatic edema, neutrophil infiltration, serum amylase and lipase levels, serum cytokine levels, and mRNA expression of multiple inflammatory mediators.
CONCLUSION: These results suggest that GJ attenuated the severity of AP as well as pancreatitis-associated lung injury.
-
Citation: Jung WS, Chae YS, Kim DY, Seo SW, Park HJ, Bae GS, Kim TH, Oh HJ, Yun KJ, Park RK, Kim JS, Kim EC, Hwang SY, Park SJ, Song HJ.
Gardenia jasminoides protects against cerulein-induced acute pancreatitis. World J Gastroenterol 2008; 14(40): 6188-6194 - URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6188.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6188
Acute pancreatitis (AP) is an acute inflammatory process of the pancreas that frequently involves peri-pancreatic tissues and remote organ systems. The severity of the disease varies widely, the clinical course is unpredictable, and specific therapy is limited[1-4]. It is generally believed that the severity of pancreatitis is determined by events that occur after acinar cell injury. Pancreatic acinar cells synthesize and release cytokines and chemokines, resulting in the recruitment of inflammatory cells such as neutrophils and macrophages. This leads to further acinar cell injury, resulting in the elevation of various pro-inflammatory mediators such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α[5,6]. The release of inflammatory mediators such as TNF-α and IL-1β during AP propagates a complex cascade of events between tissue vasculature and inflammatory cells. These inflammatory cells and mediators play a role in the systemic manifestations besides modulating pancreatic acinar cell injury; blocking the cytokine cascade in its early stage, and ameliorating the disease and its systemic complications.
Gardenia jasminoides (GJ) is widely employed in several Asian countries as a natural colorant, and has been used in Chinese traditional medicine for its homeostatic, antiphlogistic, analgesic and antipyretic effects. Its main components include geniposide and crocin[7]. These components exhibit antioxidant, cytotoxic, antitumor and neuroprotective effects[8-10]. However, the impact of GJ and its components on cerulein-induced AP have not been examined.
The present study was designed to confirm the preventive effects of GJ in a mouse model of cerulein-induced AP. In order to gain a better insight into the mechanism of action of the observed anti-inflammatory effects of GJ, we investigated the effects of GJ on (1) pancreas weight/body weight (PW/BW) ratio, (2) pancreatic histology, (3) serum amylase and lipase levels, (4) serum level of pro-inflammatory mediators, such as TNF-α, IL-1β and IL-6, and (5) lung histology.
Avidin-peroxidase and 2’-AZINO-bis (3-ethylbenzithia-zoline-6-sulfonic acid) tablets, cerulein, Tris-HCl, NaCl, Triton X-100, hexadecyltrimethylammonium bromide and etramethylbenzidine were purchased from Sigma (St. Louis, MO, USA). Anti-mouse TNF-α, IL-6 and IL-1β antibodies, recombinant TNF-α, IL-6 and IL-1β were purchased from R&D Systems (Minneapolis, MN, USA).
GJ was prepared by decocting the dried prescription of herbs with boiling distilled water. The decoction time was about 3 h. Their voucher specimens were deposited at the Herbarium at the College of Oriental Medicine, Won-Kwang University.
All experiments were performed according to protocols approved by the Animal Care Committee of the university. Female C57BL/6 mice (6-7-wk old, weighting 18-20 g) were purchased from Orient Bio Co. (Sungnam, KyungKiDo, Republic of Korea). All animals were bred and housed in standard shoebox cages in a climate-controlled room with an ambient temperature of 23 ± 2°C and a 12-h light-dark cycle for 7 d. Animals were fed standard laboratory chow, given water and randomly assigned to control or experimental groups. The mice were fasted for 18 h before induction of AP. Six mice were included in each experimental group.
AP was induced by supramaximal concentration of cerulein (50 μg/kg), a stable CCK analogue, by administering it intraperitoneally every hour for a total of 6 h as described previously[11]. The mice were fed orally with GJ (1 g/kg, 0.1 g/kg, n = 6 each) or normal saline (control group, n = 6), followed by intraperitoneal injection of cerulein (50 μg/kg) or saline every hour for a total of 5 h. The mice were sacrificed at 12 h after the completion of cerulein injections. Blood samples were obtained to determine serum amylase, lipase and cytokine levels. The pancreas was rapidly removed for morphologic examination and scoring. A portion of pancreas was stored at -70°C and prepared for the measurement of tissue myeloperoxidase (MPO) activity, an indicator of neutrophil sequestration, and for reverse-transcriptase PCR (RT-PCR) and real-time PCR measurements.
The entire pancreas of at least six mice from each treatment group was examined and semi-quantitated based on the degree of necrosis, vacuolization, inflammation, and edema. Using the previously described method of Ethridge et al[12], entire sections (a minimum of 100 fields) of pancreas were examined from each sample and scored on a scale of 0-3 (0 being normal and 3 being severe), based on the number of necrotic acinar cells, and the presence of vacuolization, interstitial edema, and interstitial inflammation. The characteristics included were: presence of acinar-cell ghosts, vacuolization and swelling of the acinar cells, and/or the destruction of the histo-architecture of the whole or parts of the acini.
ELISA for TNF-α, IL-1β and IL-6 (R&D Systems) was carried out in duplicate in 96-well plates (Nunc, Denmark), coated with each of the following: 100 μL aliquots of anti-mouse IL-6, IL-1β and TNF-α monoclonal antibodies at 1.0 μg/mL in PBS at pH 7.4, and incubated overnight at 4°C. The plates were washed in PBS containing 0.05% Tween-20 (Sigma) and blocked with PBS containing 1% BSA, 5% sucrose and 0.05% NaN3 for 1 h. After additional washes, the standards were added and incubated at 37°C for 2 h. After incubation for 2 h at 37°C, the wells were washed, and the following were added: 0.2 μg/mL of biotinylated anti-mouse TNF-α, IL-1β and IL-6, and again incubated at 37°C for 2 h. After the wells were washed, avidin-peroxidase was added and the plates were incubated for 20 min at 37°C. The wells were again washed and ABTS substrate was added. Color development was measured at 405 nm using an automated microplate ELISA reader. A standard curve was run on each assay plate using recombinant TNF-α, IL-1β and IL-6 in serial dilutions.
Arterial blood samples for determination of serum amylase and lipase were obtained 12 h after induction of pancreatitis. The mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). After anesthetization, blood samples were withdrawn from the heart. Serum amylase was measured using ADIVA 1650 (Bayer, USA). Serum lipase was measured using a Cobas-mira (Roche, USA).
mRNA transcripts were analyzed by RT-PCR in mouse pancreatic tissues. Total RNA was isolated from the mouse pancreas using Qiagen RNeasy kit and subjected to reverse transcription using SuperScript II RT (Invitrogen). Taqman quantitative RT-PCR with a 7700 Sequence Detection System was done according to the instructions of the manufacturer (Applied Biosystems). For each sample, triplicate test reactions and a control reaction lacking reverse transcriptase were analyzed for expression of the gene of interest, and results were normalized to those of the ‘housekeeping’ HPRT mRNA. Arbitrary expression units were calculated by division of expression of the gene of interest by ribosomal protein HPRT mRNA expression. The forward, reverse and probe oligonucleotide primers for multiplex real-time TaqMan PCR were as follows: for mouse TNF-α (forward, 5'-TCTCTTCAAGGGACAAGGCTG-3'; reverse, 5'-ATAGCAAATCGGCTGACGGT-3'; probe, 5'-CCCGACTACGTGCTCCTCACCCA-3'), for mouse IL-1β (forward, 5'-TTGACGGACCCCAAAAGAT-3'; reverse, 5'-GAAGCTGGATGCTCTCATCTG-3'; universal probe, M15131.1-Roche Applied Science), for mouse IL-6 (forward, 5'-TTCATTCTCTTTGCTCTTGAATTAGA-3'; reverse, 5'-GTCTGACCTTTAGCTTCAAATCCT-3'; universal probe, M20572.1-Roche Applied Science), for mouse HPRT (forward, 5'-GACCGGTCCCGTCATGC-3'; reverse, 5'-CATAACCTGGTTCATCATCGCTAA-3'; probe, 5'-ACCCGCAGTCCCAGCGTCGT-3').
Neutrophil sequestration in the pancreas was quantified by measuring the tissue MPO activity[11,12]. Tissue samples were thawed, homogenized in 20 mmol/L phosphate buffer (pH 7.4), and centrifuged (10 000 ×g, 10 min, 4°C), and the resulting pellet was resuspended in 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). The suspension was subjected to four cycles of freezing and thawing and was further disrupted by sonication (40 s). The sample was then centrifuged (10 000 ×g, 5 min, 4°C), and the supernatant used for the MPO assay. The reaction mixture consisted of the supernatant, 1.6 mmol/L tetramethylbenzidine (Sigma), 80 mmol/L sodium phosphate buffer (pH 5.4), and 0.3 mmol/L hydrogen peroxide. The mixture was incubated at 37°C for 110 s, the reaction was terminated with 2 mol/L of H2SO4, and the absorbance was measured at 450 nm. This absorbance was then corrected for the DNA content of the tissue sample (fold increase over control).
The results were expressed as means ± SE. The significance of change was evaluated using Student’s t test. Differences between the experimental groups were evaluated by using analysis of variance. Values of P < 0.05 were accepted as statistically significant.
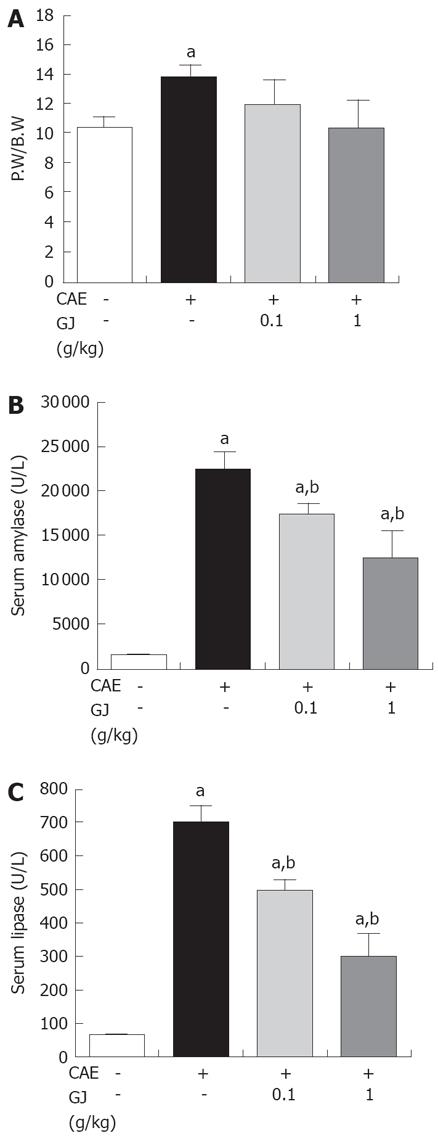
To assess the effect of GJ on the PW/BW ratio, the pancreatic weight was divided by the body weight of the mice. GJ reduced significantly the PW/BW ratio, compared with the normal saline-treated group, in a dose-dependent manner (P < 0.05) (Figure 1A). The serum levels of amylase and lipase are commonly used as markers of AP[13,14]. GJ reduced significantly the serum amylase and lipase levels in cerulein-induced AP (Figure 1B and C).
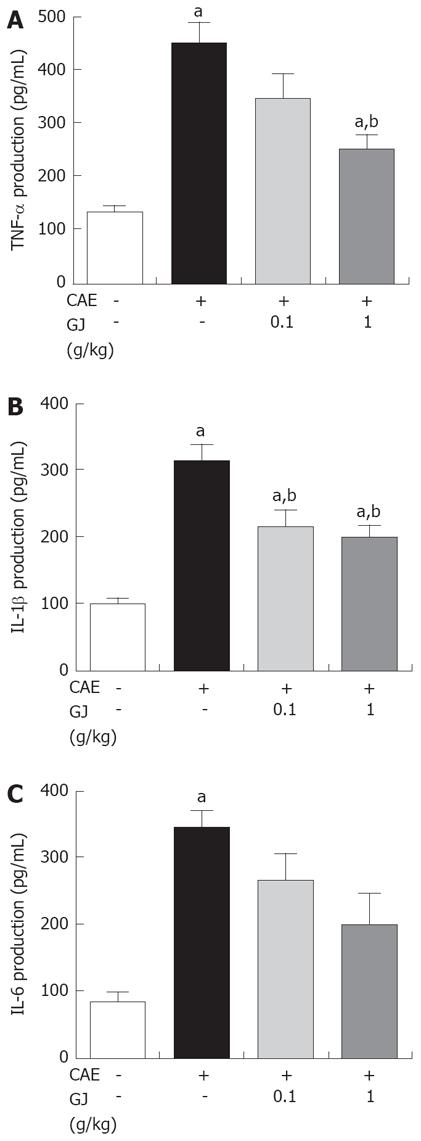
The serum levels of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6 were increased in cerulein-induced AP[15-17]. GJ decreased significantly the levels of TNF-α and IL-1β in the cerulein-induced AP. Moreover, GJ was associated with a trend towards suppression of IL-6, although the difference was not statistically significant (Figure 2).
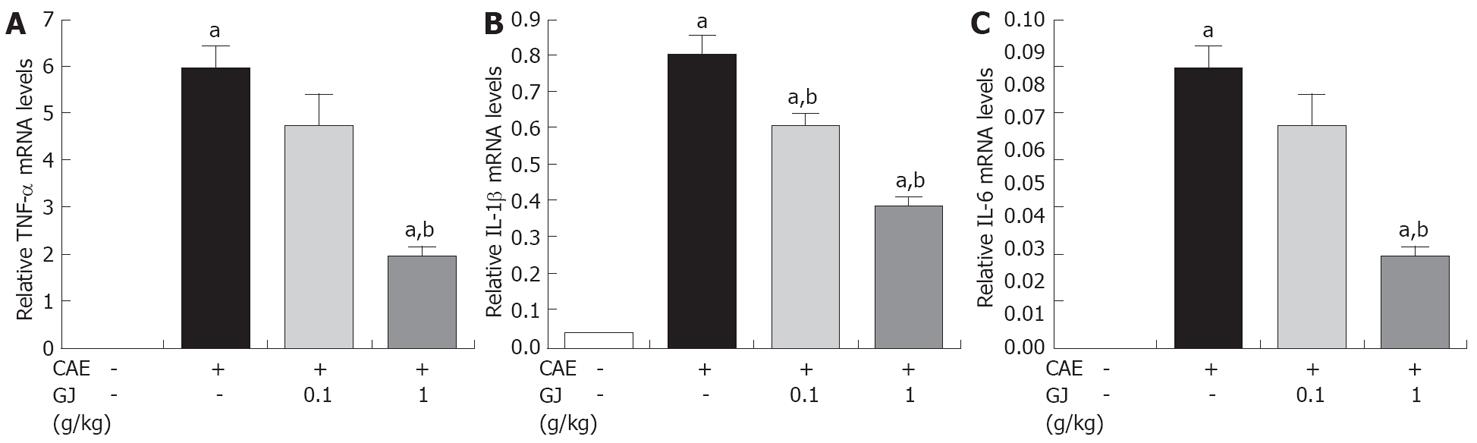
The GJ-pretreated group showed significant reduction of pancreatic tissue mRNA expression of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, in a dose-dependent manner, compared with the saline pretreated group in cerulean-induced AP (Figure 3).
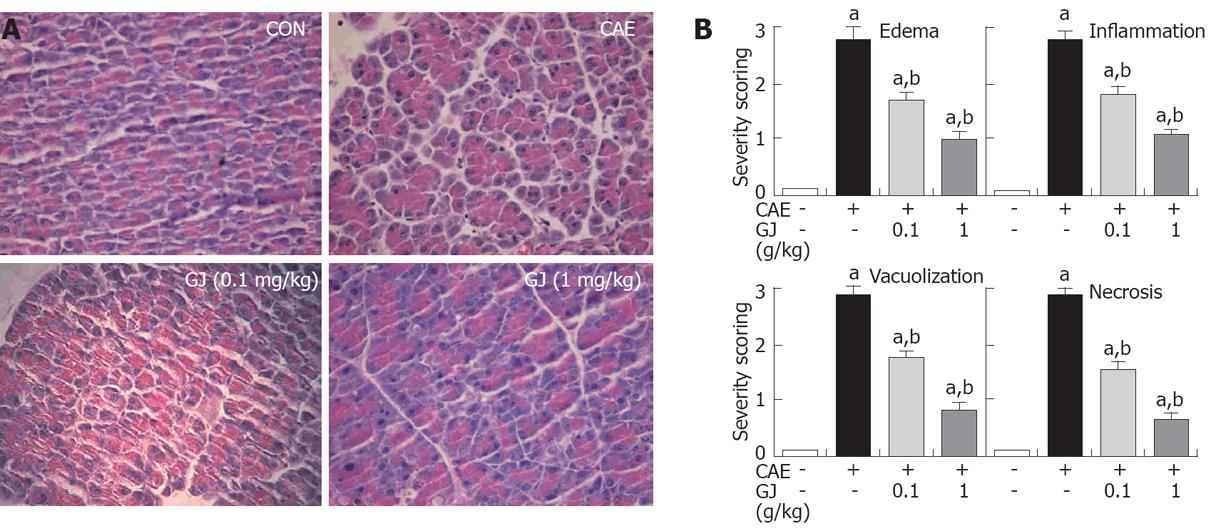
In normal mice, the histological features of the pancreas were typical of a normal architecture. Mice treated with i.p. injections of cerulein developed acute necrotizing pancreatitis. Histological examination of the pancreas (at 12 h after the injection of cerulein) revealed tissue damage characterized by inflammatory cell infiltrate and acinar cell necrosis. GJ pretreatment resulted in significant reduction in pancreatic injury (Figure 4A). The presence of edema, inflammation, vacuolization, and necrosis were reduced significantly in the GJ-pretreated group compared with the normal saline-pretreated group, in a dose-dependent manner (Figure 4B).
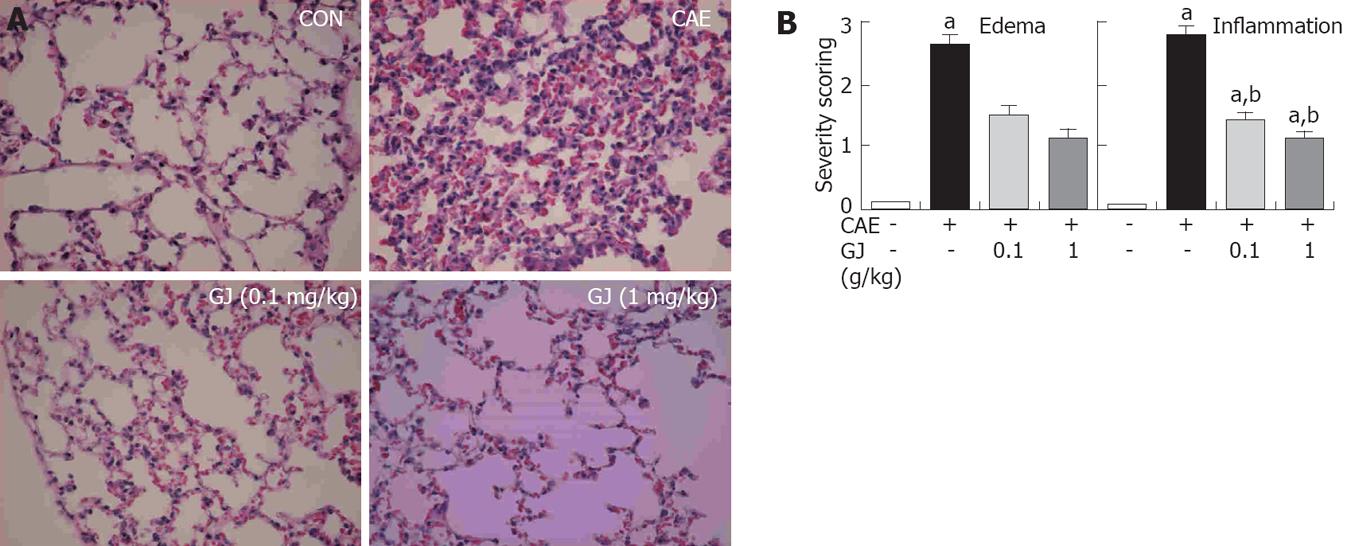
Lung injury commonly develops early in AP. AP-associated lung injury is characterized by edema and inflammation[18]. In addition to the pancreas, we assessed the lungs after cerulein administration. Histological examination of lung sections (at 12 h after the injection of cerulein) revealed tissue damage characterized by edema and inflammatory cell infiltrate. GJ pretreatment resulted in significant reduction in lung injury. The histological sections were scored for edema and inflammation. The lungs of the GJ-pretreated mice had significantly less edema and inflammation compared with lungs from saline-injected control animals (Figure 5).
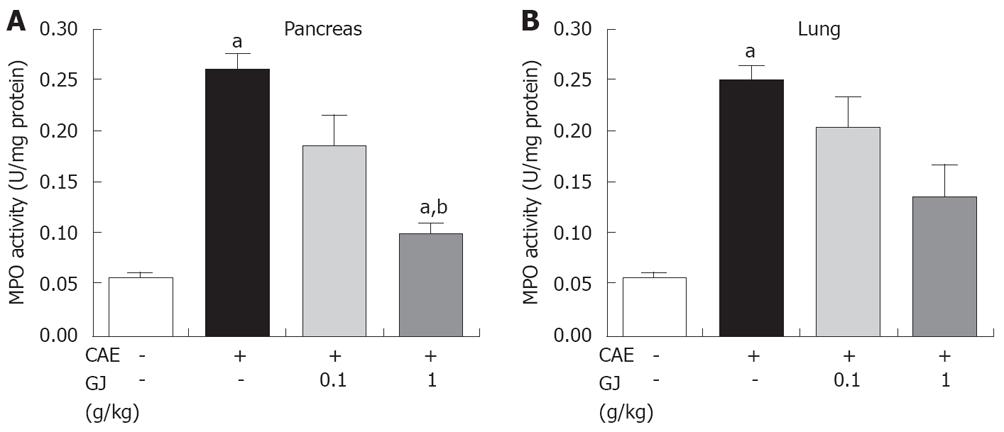
As an additional quantitative assessment of the severity of the inflammatory response, we measured MPO activity, an indicator of neutrophil sequestration, in the pancreas and lung following induction of AP in the GJ-pretreated mice and saline-injected control animals. MPO activity in the pancreas and lung in the GJ-pretreated mice was significantly less compared with the saline-injected control animals (Figure 6).
AP is associated with a high rate of morbidity and mortality. The mortality rate in patients with severe AP is as high as 20% to 30%[1,2]. AP, characterized by interstitial edema, vacuolization, inflammation and acinar cell necrosis, is commonly caused by excessive ethanol consumption, biliary tract disease, certain medications, and invasive procedures of the biliary and pancreatic ducts[19-22]. The pathophysiology of AP is poorly understood, and the clinical course is unpredictable[7].
The fruit of GJ has been used as an oriental herbal medicine in traditional formulations. It has been employed in the treatment of inflammation, jaundice, headache, edema, fever, hepatic disorders, and hypertension. In addition, the pigments obtained from the fruit are used as food colorants. The pharmacologic actions of GJ, such as protective effect against oxidative damage, cytotoxic activity, anti-inflammatory actions, and fibrolytic activity have been described in detail[23,24]. The present study was carried out to determine whether GJ could inhibit the severity of cerulein-induced AP.
Several markers of AP such as PW/BW, amylase and lipase activity were reduced (Figure 1). There is much evidence to implicate the involvement of inflammatory mediators, such as cytokines, TNF-α, IL-1β, and IL-6 in the development of pancreatitis[25]. In experimental pancreatitis, the serum levels of TNF-α and IL-1β are elevated and their blockade attenuates the disease process[18,25]. IL-6 is one of the principal cytokine mediators of the acute-phase response, and has been suggested as a marker for predicting the severity of AP[25]. As shown in Figures 2 and 3, GJ may inhibit these cytokines in AP.
The results of the present study indicate that cerulein caused significant morphological abnormalities in the pancreas, as demonstrated by the appearance of vacuolization, inflammatory infiltration and changes in histo-architecture of the pancreatic acini. Pretreatment with GJ inhibited acinar cell death as well as infiltration by inflammatory cells in cerulein-induced AP. To rule out interference by the binding of cerulein to CCK receptors on pancreatic acinar cells, we examined the effect of GJ in pancreatic acinar cells. GJ itself did not have any cytoxicity at 6 h. However, GJ inhibited cerulein-induced aciniar cell death in a dose-dependent manner (data not shown).
Lung injury commonly develops early in AP. AP-associated lung injury is characterized by edema and inflammation[12]. Therefore, we also assessed the lungs after cerulein administration. Histological examination of lung sections (at 12 h after the injection of cerulein) revealed tissue damage characterized by edema and inflammatory cell infiltrate. GJ pretreatment resulted in a significant reduction in lung injury. The histology sections were scored for edema and inflammation and, as shown in Figure 5, the lungs of the GJ pretreated mice had significantly less edema and inflammation compared with lungs from the saline treated control animals.
In conclusion, the present study shows that GJ pretreatment ameliorated the severity of cerulein-induced AP in rats. Additionally GJ pretreatment ameliorated many of the laboratory and biochemical parameters of the disease. Our findings suggest that GJ may be beneficial in the treatment of acute pancreatitis.
Peer reviewer: James H Grendell, Professor of Medicine, Chief-Division of Gastroenterology, Hepatology, & Nutrition, Winthrop University Hospital, 222 Station Plaza N. #429, Mineola, New York 11501, United States
S- Editor Tian L L- Editor Anand BS E- Editor Lin YP
| 1. | Banks PA. Medical management of acute pancreatitis and complications. The pancreas: biology, pathobiology, and disease. New York: Raven 1993; 593-611. |
| 2. | Rau B, Uhl W, Buchler MW, Beger HG. Surgical treatment of infected necrosis. World J Surg. 1997;21:155-161. |
| 3. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. |
| 4. | Ho HS, Frey CF. Gastrointestinal and pancreatic complications associated with severe pancreatitis. Arch Surg. 1995;130:817-822; discussion 822-823. |
| 5. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. |
| 6. | Lane JS, Todd KE, Gloor B, Chandler CF, Kau AW, Ashley SW, Reber HA, McFadden DW. Platelet activating factor antagonism reduces the systemic inflammatory response in a murine model of acute pancreatitis. J Surg Res. 2001;99:365-370. |
| 7. | Hu Z, Wang Y, Luo G, He L. [Estimation of measurement uncertainty of analytical results for the determination of three active components from Gardenia jasminoides Ellis. by HPLC]. Zhong Yao Cai. 2005;28:991-994. |
| 8. | Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116-122. |
| 9. | Pham TQ, Cormier F, Farnworth E, Tong VH, Van Calsteren MR. Antioxidant properties of crocin from Gardenia jasminoides Ellis and study of the reactions of crocin with linoleic acid and crocin with oxygen. J Agric Food Chem. 2000;48:1455-1461. |
| 10. | Oh PS, Lim KT. Plant originated glycoprotein has anti-oxidative and anti-inflammatory effects on dextran sulfate sodium-induced colitis in mouse. J Biomed Sci. 2006;13:549-560. |
| 11. | Van Acker GJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G794-G800. |
| 12. | Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311-1322. |
| 13. | Strowski MZ, Sparmann G, Weber H, Fiedler F, Printz H, Jonas L, Goke B, Wagner AC. Caerulein pancreatitis increases mRNA but reduces protein levels of rat pancreatic heat shock proteins. Am J Physiol. 1997;273:G937-G945. |
| 14. | Tietz AB, Malo A, Diebold J, Kotlyarov A, Herbst A, Kolligs FT, Brandt-Nedelev B, Halangk W, Gaestel M, Goke B. Gene deletion of MK2 inhibits TNF-alpha and IL-6 and protects against cerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1298-G1306. |
| 15. | Exley AR, Leese T, Holliday MP, Swann RA, Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992;33:1126-1128. |
| 16. | Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97-117. |
| 17. | Ranson JH, Turner JW, Roses DF, Rifkind KM, Spencer FC. Respiratory complications in acute pancreatitis. Ann Surg. 1974;179:557-566. |
| 18. | Gross V, Leser HG, Heinisch A, Scholmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522-530. |
| 19. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. |
| 20. | Kaiser AM, Saluja AK, Steer ML. Repetitive short-term obstructions of the common bile-pancreatic duct induce severe acute pancreatitis in the opossum. Dig Dis Sci. 1999;44:1653-1661. |
| 21. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. |
| 22. | Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41-45. |
| 23. | Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97:61-67. |
| 24. | Jagadeeswaran R, Thirunavukkarasu C, Gunasekaran P, Ramamurty N, Sakthisekaran D. In vitro studies on the selective cytotoxic effect of crocetin and quercetin. Fitoterapia. 2000;71:395-399. |