Published online Oct 28, 2008. doi: 10.3748/wjg.14.6180
Revised: June 2, 2008
Accepted: June 9, 2008
Published online: October 28, 2008
AIM: To evaluate measurements of intragastric pH with the Bravo capsule system over a prolonged time.
METHODS: A Bravo capsule was placed inside the rat gastric body and pH was studied for periods up to five consecutive days. For comparison, a gastric fistula model was used. Effects of ghrelin and esomeprazole, with or without pentagastrin, on gastric pH were studied. In addition, effects of esomeprazole on plasma ghrelin, gastrin and somatostatin were analyzed.
RESULTS: All rats recovered after surgery. The average 24-h pH during free feeding was 2.3 ± 0.1 (n = 20) with a variation of 18% ± 6% over 5 d. Ghrelin, 2400 pmol/kg, t.i.d. increased pH from 1.7 ± 0.1 to 3.1 ± 0.3 (P < 0.01) as recorded with the Bravo system. After esomeprazole (1 mg/kg, 3 mg/kg and 5 mg/kg) there was a dose-dependent pH increase of maximally 3.4 ± 0.1, with day-to-day variation over the entire period of 8% ± 3%. The fistula and pH studies generated similar results. Acid inhibition with esomeprazole increased plasma ghrelin from 10 ± 2 pmol/L to 65 ± 26 pmol/L (P < 0.001), and somatostatin from 10 ± 2 pmol/L to 67 ± 18 pmol/L (P < 0.001).
CONCLUSION: pH measurements with the Bravo capsule are reliable, and comparable to those of the gastric fistula model. The Bravo system optimizes accurate intragastric pH monitoring over prolonged periods and allows both short- and long-term evaluation of effects of drugs and hormones.
- Citation: Rudholm T, Hellström PM, Theodorsson E, Campbell CA, McLean PG, Näslund E. Bravo capsule system optimizes intragastric pH monitoring over prolonged time: Effects of ghrelin on gastric acid and hormone secretion in the rat. World J Gastroenterol 2008; 14(40): 6180-6187
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6180.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6180
In the past, different techniques have been employed to study gastric acid secretion in rodents. The main principle for these methods has been collection of gastric juice, and in order to measure acid secretion, pH titration has been carried out. One of the earliest methods was the pylorus ligation technique[1]. The principle of this method is distension of the stomach as a potent stimulus of acid secretion. Later, this method was altered with an esophageal ligature[2,3], after which the stomach of the rat was removed and secretions analyzed. Esophageal ligation in the pylorus-ligated rat has been shown to significantly inhibit acid secretion by inhibition of central vagus function[2]. Since then, the most reliable method has been the chronic fistula method[4-6] where a gastric fistula is implanted at the greater curvature of the stomach. This technique requires movement restriction of the animal which is in a conscious state during the study. The gastric contents are collected and acid output measured. This technique allows re-use of animals following a recovery period from the experimental procedure. Other methods used today are perfusion of the gastric lumen[7] and isolated perfused, as well as vascularly perfused rat stomach[8-10].
Most of the above studies have the drawback that they do not measure intragastric pH directly and are not very physiological, as the animal is either restrained or anesthetized. The main goal of this study was to test the feasibility of a capsule normally used in the clinical setting in humans to measure gastroesophageal reflux disease (Bravo system) for monitoring intragastric pH in the rat. The Bravo capsule system has primarily been used in humans[11-14], but also in animals[15] for diagnosis of gastroesophageal reflux disease.
The aim of the study was to evaluate the Bravo capsule for pH monitoring in the rat. To validate the method, we compared the data to those of the standard gastric fistula model.
Sprague-Dawley male rats (300-350 g) were purchased from Scanbur B&K AB (Sollentuna, Sweden). The rats were housed in wire-meshed cages at 24°C with constant humidity and 12:12 h light-dark cycle. The animals were fed ad libitum with a commercial rat diet consisting of pellet (LABFOR, Lactamin R36, Kimstad, Sweden) and tap water prior to the studies. The experiments were approved by the Animal Ethics Committee in northern Stockholm.
Surgery was performed under anesthesia with pentobarbital sodium (50 mg/kg; Apoteket AB, Stockholm, Sweden) intraperitoneally, and Hypnorm (fentanyl citrate, 0.315 mg/kg and fluanisone 10 mg/kg; Janssen, Oxford, USA) intramuscularly. Marcain (bupivacaine hydrochloride, 2.5 mg/kg; AstraZeneca, Södertälje, Sweden) was given subcutaneously after surgery along the abdominal incision.
For the Bravo system studies, a midline incision was performed, and a small opening created in the proximal greater curvature, and gastric contents were evacuated. An externally pre-calibrated (buffers pH 1.07 and 7.1) Bravo capsule (an electronic sensor encapsulated in PVC-plastic, 25 mm × 5 mm × 5 mm; Synmed Medicinteknik AB, Spånga, Sweden) was placed inside the stomach with the pH sensor pointing distally and anchored with a suture. An indwelling silastic catheter (Dow Corning Co., Midland, MI, USA) was inserted into the external jugular vein.
For the gastric acid fistula studies, rats were provided with a plastic gastric fistula placed immediately proximal to the oxyntic gland area near the greater curvature. The fistula was closed between experimental periods. A silastic catheter was implanted into the external jugular vein for drug administration.
Studies of intragastric pH began in the morning 2 d after surgery. The studies were carried out in conscious rats, one experiment for each rat, under normal conditions, or after a 16-h fasting period in wire-bottom cages with free access to water. The animals gained weight (10 ± 3.4 g during 1 wk) and behaved in a normal fashion, with a normal feeding pattern throughout the experiments. At post-mortem examination, no mucosal lesions, obstruction of the pylorus or gastric distension were seen. Drugs were administered through the external jugular vein in all experiments.
The pH recorded by the Bravo capsule was transmitted to the Bravo receiver placed directly outside the cage. The sampling frequency was 6 Hz. The Bravo system was set for a 48-h registration period, after which the data were downloaded, batteries replaced and recording continued. This procedure was then repeated in two more 48-h periods.
All test compounds were dissolved and diluted in isotonic saline solution (sodium chloride, 9 g/L; 300 mosm/kg H2O, Fresenius Kabi, Halden, Norway).
The effect of ghrelin on pH: The effect of ghrelin on intragastric pH was studied with ghrelin (2400 pmol/kg) given t.i.d (08:00, 12:00 and 16:00) for 5 d in a row (n = 7).
Evaluation of basal pH: Baseline pH was studied over 24 h under fed (n = 20) and fasting (n = 8) conditions.
Effect of esomeprazole on pH: The effect of increasing bolus doses of esomeprazole (AstraZeneca) (1 mg/kg, 3 mg/kg or 5 mg/kg iv, n = 10) or saline (iv, n = 8) was studied for 24 h in fed rats. Furthermore, the effect of esomeprazole (3 mg/kg iv, n = 10) or saline (iv, n = 8) was studied for 24 h in fasting rats.
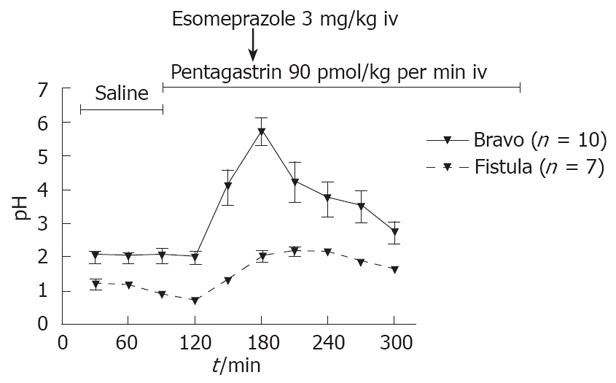
The effect of pentagastrin and esomeprazole on pH: The effect of esomeprazole (3 mg/kg iv, n = 10) or saline (iv, n = 8) was studied under pentagastrin (NeoMPS, Strasbourg, France) infusion (90 pmol/kg per min, iv) over 6 h in both fed and fasting rats. In these experiments, the rats were restrained in Bollman cages to mimic the gastric fistula studies and for infusion of pentagastrin.
The effect of a 24-h infusion of pentagastrin (90 pmol/kg per min iv, n = 6), of esomeprazole (9 pmol/kg per min, n = 6), or saline (0.154 mol/L, n = 6) on pH was studied.
Plasma levels of gut hormones: The effect of esomeprazole (3 mg/kg iv) on plasma levels of ghrelin, gastrin and somatostatin was studied. A group of animals (n = 10) was divided into two treatment groups (each n = 5). All animals were treated with esomeprazole daily during 1 wk. The first group of animals was then euthanized, while the other group was followed for another week without esomeprazole and then euthanaized. Blood was drawn and centrifuged, and plasma assayed for concentrations of ghrelin, gastrin and somatostatin.
For ghrelin measurements, the ghrelin (active) radioimmunoassay kit (Linco Research, St. Charles, MI, USA) was used, which utilizes 125I-labeled ghrelin and ghrelin antiserum to determine the level of active ghrelin in plasma. For the analysis, a Gamma Master 1277 (LKB-Wallac, Perkin-Elmer Inc, Massachusetts, NH, USA) was used. The intra- and interassay coefficients of variation were 7% and 14%, respectively.
Somatostatin was analyzed using an EIA kit (EK-060-03) from Phoenix Pharmaceuticals, Burlingame, CA, USA), which reacts 100% to somatostatin-14 and somatostatin-28. The intra- and interassay coefficients of variation were 5% and 14%, respectively.
Gastrin was analyzed using C-terminal-directed CCK/gastrin antiserum 2609/10 (Rehfeld, 1978). Chloramine-T-labeled and HPLC-purified gastrin-17 (NeoMPS) was used as radioligand and gastrin-17 as calibrator/standard. The intra- and interassay coefficients of variation were 6% and 8%, respectively.
Studies of gastric acid secretion began 7 d after surgery. The animals gained weight (8 ± 2.6 g during 1 wk) and had normal behavior during the experimentation periods. Prior to each experiment, food was withheld for 18 h, but with free access to water. At the start of the experiments, the stomach was rinsed with 10-15 mL luke-warm tap water to evacuate remaining food, followed by a 30-min period before the experiments were started. During the experiments, the conscious rats were placed in Bollman cages. Gastric juice was collected at 30-min intervals, and volumes measured to the nearest 0.1 mL. pH was calculated by back-titration using 0.1 mmol/L sodium hydroxide. Acid output was calculated by multiplying the secretion volumes with hydrogen ion concentrations and expressed as μmol per 30-min period.
Baseline acid secretion was studied for 60 min followed by esomeprazole (3 mg/kg iv), after which acid secretion was studied for another 2 h. During the experiment, saline was administered in the same amount as collected from the gastric fistula to compensate for the volume loss during the experiment. Furthermore, baseline acid secretion was studied for 60 min, followed by an infusion of pentagastrin (90 pmol/kg per min) for 4 h. After 1 h of pentagastrin infusion, a bolus of esomeprazole (3 mg/kg iv) was administered and acid secretion studied for another 3 h.
The data obtained with the Bravo capsule analyzed using (POLYGRAM NET™ pH Testing Application software, Synmed Medicinteknik) in 48-h periods. Results of studies with esomeprazole were analyzed by calculating changes in pH at various timepoints from baseline (defined as 0.5 h prior to onset of studies). For analysis of the fistula studies, the first 30-min collection was discarded and the second collection used as baseline for comparison with esomeprazole and pentagastrin.
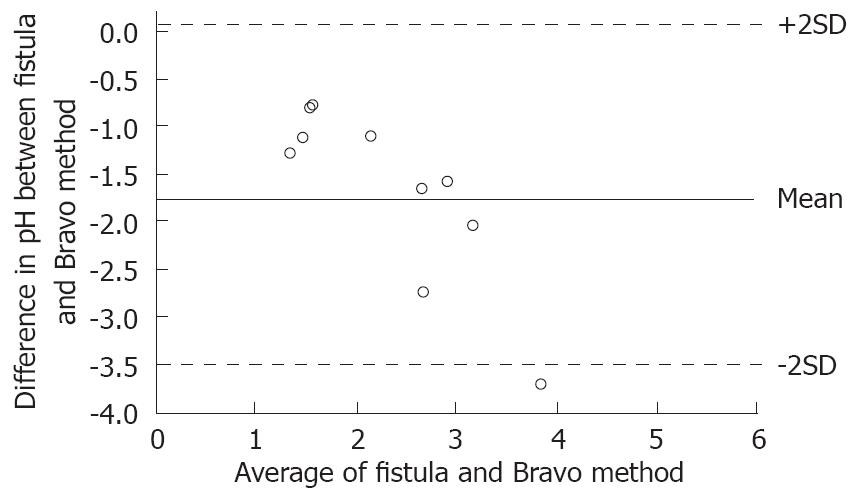
All data are mean ± SE. A Kruskal-Wallis test followed by Mann-Whitney U test was used for statistical comparisons using specific time points for pH. P < 0.05 was considered statistically significant. For comparison of the variability between the fistula and the Bravo system the Bland-Altman analysis was used[16,17]. The Prism software package 4.0 (GraphPad Software Inc., San Diego, CA, USA) was used for the statistical comparisons.
Pentagastrin resulted in a marked increase, 83 ± 9 mmol/L to 132 ± 8 mmol/L (P < 0.05) of acid output in the fistula model, which was not evident as a corresponding decrease in pH with the Bravo system. During esomeprazole treatment, there was a marked increase in pH from 2.0 ± 0.2 to 3.7 ± 0.5, as recorded with the Bravo system and correspondingly, a marked decrease in acid secretion from 105 ± 21 mmol/L to 31 ± 7 mmol/L in the fistula model (P < 0.05; Figure 1). Bland-Altman analysis of these conditions showed a high degree of agreement between the Bravo system and the fistula method as shown in Figure 2.
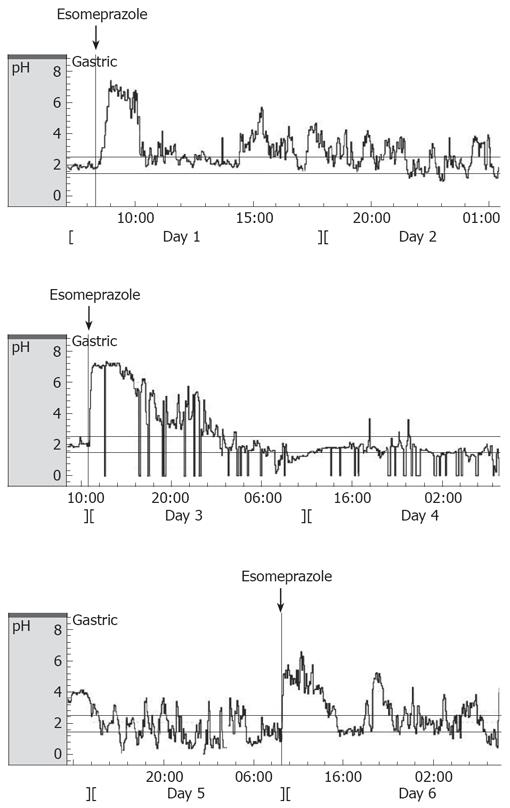
A typical 120-h baseline registration including dose of esomeprazole (day 1, 3 and 5) with the Bravo system is shown in Figure 3. The feeding status did not alter the mean pH over 24 h, but increases in pH were observed during afternoon and night-time when animals were fed. The mean 24-h pH was 2.3 ± 0.1 during fed conditions and 2.5 ± 0.3 during fasted conditions, with 18% ± 6% variation during the next four 24-h periods. There was no difference in pH between daytime and night-time (1.4 ± 0.1 and 1.7 ± 0.2, respectively).
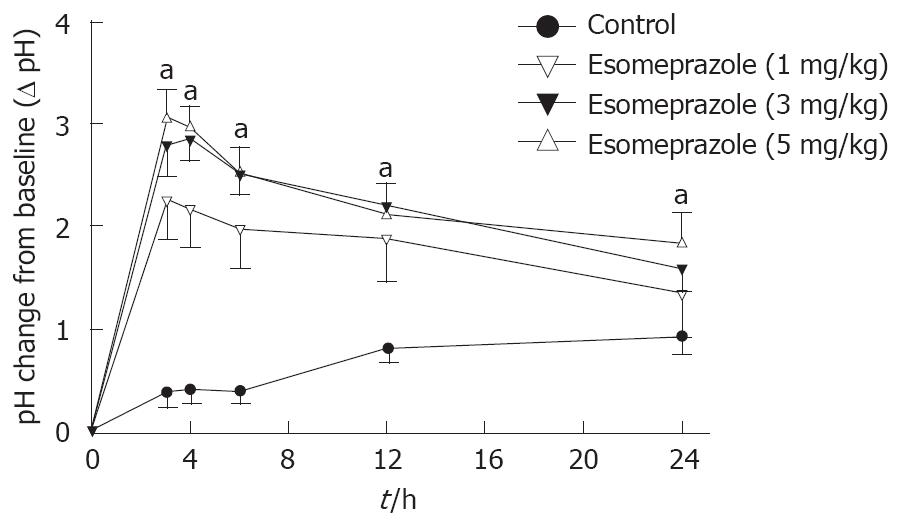
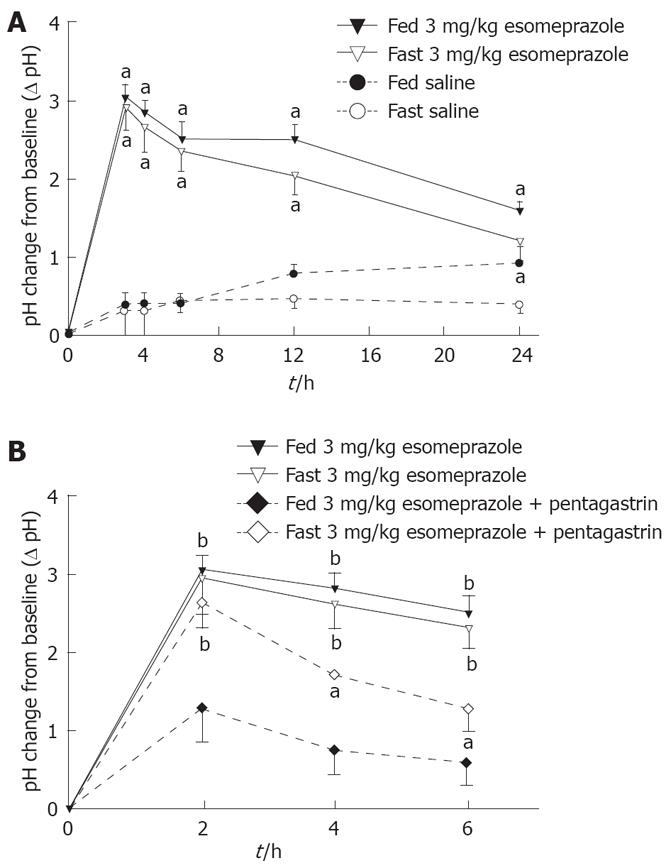
As studied over 24 h, there was a dose-dependent increase of pH after esomeprazole, 1 mg/kg, 3 mg/kg, and 5 mg/kg, during free roaming conditions (Figure 4). Already 3 h after administration of esomeprazole, pH was significantly higher with 5 mg/kg, 3.1 ± 0.4, than with 1 mg/kg, 2.2 ± 0.4 (P < 0.05). Esomeprazole (3 mg/kg) increased intragastric pH during saline infusion over a 6-h period (2.5 ± 0.2) compared to baseline pH (1.6 ± 0.2), whereas saline did not (P < 0.01; Figure 5A).
Esomeprazole was equally effective during fed or fasting conditions (Figure 5A). As a control, saline did not change intragastric pH during either fed (baseline pH 1.4 ± 0.1) or fasting (baseline pH 1.6 ± 0.2) conditions (Figure 4, Figure 5A).
Pentagastrin alone did not change pH over 6 h compared with fed (baseline pH 2.1 ± 0.2) or fasting (baseline pH 2.4 ± 0.2) conditions. After esomeprazole (3 mg/kg), pentagastrin infusion markedly decreased pH from 2.0 ± 0.3 to 1.0 ± 0.2 (P < 0.05, Figure 5B). This effect was most marked in fed animals.
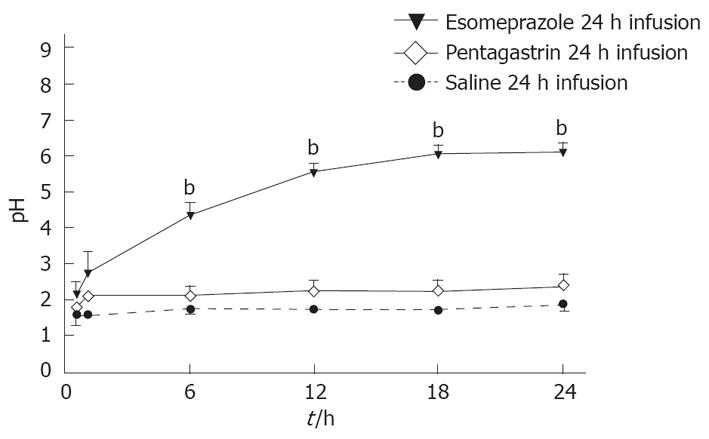
After esomeprazole (9 pmol/kg per min) the average 24-h pH was substantially higher than in the controls, 5.7 ± 0.3 and 2.1 ± 0.2, respectively (P < 0.01). Pentagastrin alone did not change pH over the 24-h infusion period as compared to saline (Figure 6).
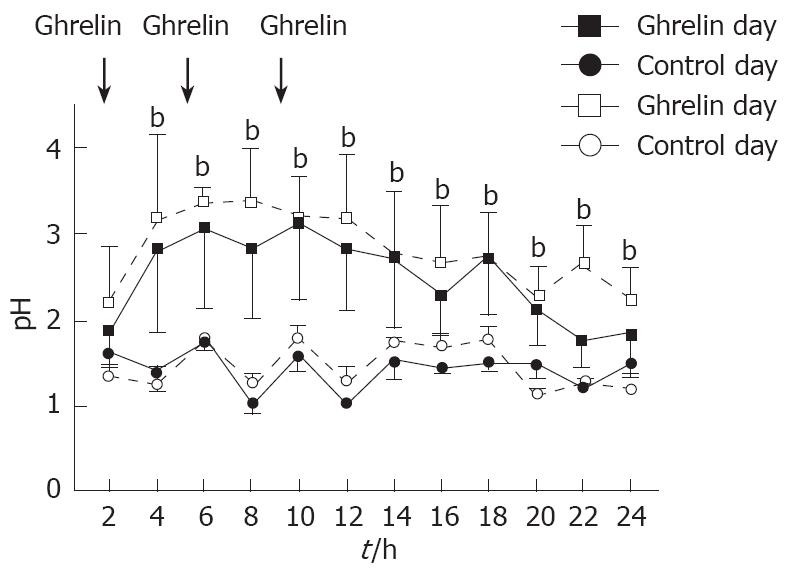
Administration of ghrelin, t.i.d markedly increased gastric 24-h pH from day 1 (2.5 ± 0.6) to day 5 (2.8 ± 0.5) compared to control day 1 (1.4 ± 0.1) and day 5 (1.5 ± 0.2) (P < 0.01; n = 7). There was no significant day-to-day variation of the ghrelin effect during the five days (Figure 7).
This study demonstrates that the Bravo system can be used for studies of intragastric pH in rats and that the results are comparable to those of a standard fistula model. The system allows for long-term studies during unrestrained living conditions. There are several advantages with the use of the Bravo system. Previous models for studies of gastric acid secretion do not allow measurements of pH over a long time. Furthermore, during these studies, the animals are kept under stressful conditions, which to a certain degree, may influence the responsiveness of the animals to different stimuli. The Bravo system uses a telemetric system that records gastric pH during 24 h for up to five consecutive days. The day-to-day variation was within acceptable limits. The system allows for real-time recordings of intragastric pH with the ability to record from the start of a treatment until a detectable effect is seen. The system is suitable for long-term studies with continuous infusions that are difficult to perform using the fistula model, as the animals do not tolerate being restrained in cages during prolonged studies. The Bravo recording system is also a digital recording system, which means that primary data are logged, and permits detailed measurements as determined by the set sampling frequency.
The data are, however, limited to pH-values as no secretion volumes are obtained. With the gastric fistula model, recordings are made over no less than 15-min periods, which can be a limiting factor as regards rapid changes in pH, i.e. drug effects. However, in the fistula model, secretion volumes are recorded, which permit calculation of a true acid output. The Bravo system has a few drawbacks. It is expensive, the battery life of the capsule is short (5 d) and therefore, the animals can only be used in studies for about a week. This means that experiments must start immediately after the operation (in this case 2 d after the surgical procedure), and the recovery from surgery may influence the results and the comparison with the fistula model. Despite this, the Bravo system seems to be well tolerated, as the stomach of the rats did not show any abnormalities or mucosal lesions upon autopsy. The animals also gain weight and behaved in a normal fashion during the experiments.
From a physiological viewpoint, our results demonstrated expected results; intragastric pH in rodents was stable over time, with a slight increase during the night during fed conditions.
In addition, treatment with esomeprazole and pentagastrin gave expected results. The agreement between the Bravo system and the established fistula method was evaluated employing a Bland-Altman analysis. When the two methods were compared, the pH results obtained with the Bravo system were comparable to those obtained using the fistula model. The differences lie within acceptable limits of agreement approximately 95% of the time, and the variability was consistent across the graph; the scatter around the baseline (mean) did not increase with increasing means.
During comparative studies, the animals were restrained in Bollman cages for infusion of pentagastrin, so the experimental conditions were the same. During esomeprazole treatment, pH rose and gastric acid output decreased accordingly. There seemed to be a slight delay in response to esomeprazole when studied by the fistula method as compared to the Bravo system. The reason for this is probably related to the fact that the secretory response depends on the physical emptying of gastric contents from the fistula until measurements can be done. As judged from our experiments, this causes a delay of the recorded response of about 30 min. Pentagastrin increased acid output, but no change was seen in intragastric pH with the Bravo system. This is explained by the fact that a change in secretion volume does not affect the pH recorded, even though acid output is changed. The fact that pH does not change when introducing pentagastrin may be due to the constantly low basal pH level in the rat stomach.
The gut hormones assayed in this study, ghrelin, gastrin, and somatostatin, are all found in the mucosa of the stomach[18]. They operate in a coherent inhibitory/stimulatory fashion against one another, i.e. increasing levels of somatostatin stimulates ghrelin, while gastrin is inhibited[19,20]. Pentagastrin acts as an agonist on acid secretion and has a stimulatory effect on somatostatin, which in turn down-regulates the release of gastrin so that excessive amounts of acid are not produced[20]. The fact that basal plasma gastrin levels remained stable with the Bravo system indicates that the Bravo capsule by itself does not distend the stomach to such a degree that gastrin levels are affected[21].
Our results using the Bravo system, with an increase of intragastric pH during 1 wk after three times daily, administration of ghrelin, are in accordance with earlier studies[19,22], but at variance with another[23]. This may be explained by the fact that different methods for studying gastric acid secretion have been employed, some of which are dependent on gastric motility for the emptying of gastric secretions through the fistula. By using the Bravo system, we found no desensitization of the pH response to ghrelin. This is at variance with our previous studies on intestinal motility, in which a loss of the ghrelin response was shown[19,24]. This might be due to the fact that motility was stimulated by a continuous infusion of the hormone, whereas the pH effect was brought about by repeated injections of ghrelin, a form of administration that is considered less liable to desensitization effects. As ghrelin not only increases intragastric pH, but also stimulates gastric emptying in rodents[22,23,25,26]. This may be an erroneous factor in determining acid secretion using the fistula method.
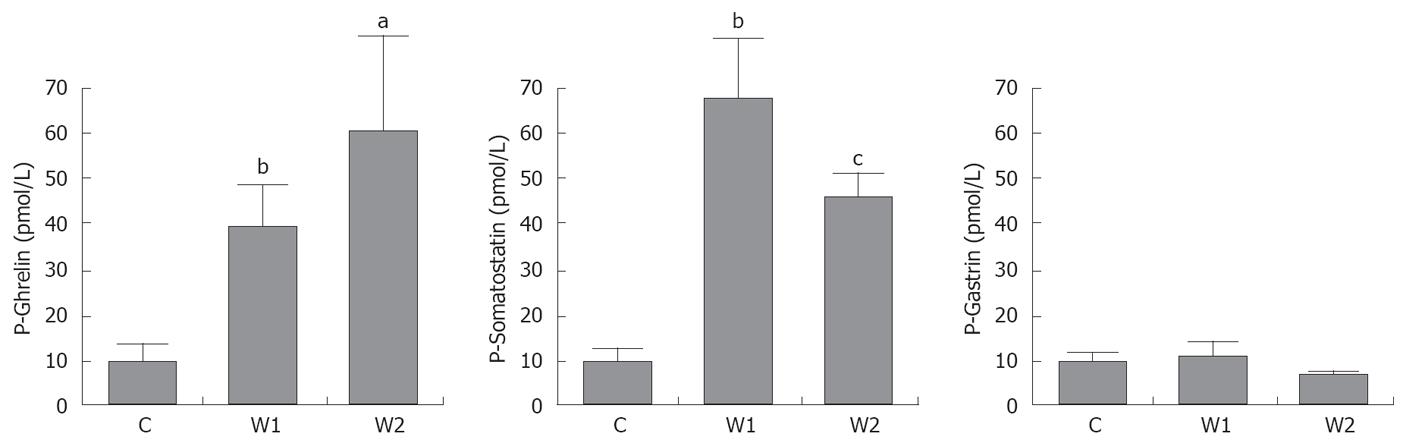
With esomeprazole treatment, plasma concentrations of ghrelin and somatostatin were increased. This effect was maintained for 1 wk after esomeprazole treatment. The underlying mechanism for this increase in plasma ghrelin and somatostatin is not yet fully understood, but may be due to a direct effect of esomeprazole on ghrelin and somatostatin, but also by an indirect effect through changes in gastric pH. The counter-balancing effects between pentagastrin (low pH) and esomeprazole (high pH) as regards ghrelin levels point to a physiological role of ghrelin in the control of gastric acid secretion[27,28]. The rise in somatostatin concentration is likely due to a direct effect of the continuous doses of ghrelin, as pH was not affected. The lack of elevated levels of gastrin for the two groups are probably attributed to the increase in somatostatin[29] or, although less likely, low doses of esomeprazole[30,31].
To conclude, the Bravo capsule system is to be used for prolonged studies of gastric pH in free roaming conscious rats over days and is well tolerated, and could serve as a complement to the gastric fistula model, as shown by acid and gut hormone secretion measurements.
The pharmacological treatment of gastrointestinal acid-related diseases aims at providing ulcer and mucosal healing, symptom relief and improved quality of life. Gastric acid inhibitory compounds are widely used in the clinical setting in order to treat not only benign gastric and duodenal ulcers, but also gastritis and reflux esophagitis. Over the past two decades, there has been a number of reports on the use of proton pump inhibitors (PPIs) such as omeprazole and the following competitors. The PPIs are activated in the acid environment in the stomach and inhibit the final step of gastric acid secretion. They bind in a non-competitive way to the H+, K+ -ATPase and inhibit secretion. Even though the PPIs have many good properties compared to other treatment regims, and are considered the treatment of choice in acid-related gastrointestinal diseases, there are drawbacks with PPI treatment. For instance, the onset of action is slow as compared to that of H2-receptor antagonists, which induce an immediate acid inhibition, and the duration of action may be too short giving room for night-time acid breakthrough. So far, treatments have got around this problem by recommending a two-dose regimen. Pharmaceutical development has been directed against finding a compound with profound acid inhibitory action over prolonged periods of time, not permitting night-time acid breakthrough to take place. The development of such drugs, however, require new methods of studying gastric acid secretion over prolonged periods, up to 120 h over or more.
Research concerning acid-related diseases has been focused on PPIs targeted against the H+, K+ -ATPase of the stomach and H2-receptor antagonists. Recent studies have shown that the proton pump is the most likely candidate for a sustainable therapeutic application in the regulation of acid suppression. One of the hurdles in this field is the possibility to perform long-term measurements of acid secretion in the development of pharmacological treatment of acid diseases. Although PPIs are highly effective as a class, differences in their pharmacokinetics, such as bioavailability, metabolism, and elimination half-life, may translate into differences in clinical outcomes.
Over the latest years, new drugs have emerged on the market, such as being PPIs (3rd generation), new potassium channel blocking agents that inhibit gastric secretion (P-CAP), and even combinations of PPIs and H2-receptor antagonists. A second line to this further development is to be expected and with this new method, developed as a tool for evluation of such long-acting drugs, may become a feasible tool in the clinical setting for treatment of acid-dependent diseases.
Our research demonstrates stable recordings with the Bravo capsule system in the rat. The animals were given PPI and ghrelin and this resulted in an almost immediate response in pH, sustained during approximately 6 h. The capsule model was compared with the fistula model and showed agreement in compliance between the two methods. This indicates that the capsule model could eventually replace the fistula model. It seems better to use the former method because of less strain on the rats, and easier and more gentle handling and experimental procedures. Furthermore, the Bravo system set-up is easy to manage and the information recorded allows many different analysis variables. The system also records over five consecutive days, which previously has not been possible in this setting.
Bravo capsule system: A catheter-free system used to measure esophageal pH (acidity) levels in patients who have or are suspected of having gastroesophageal reflux disease, but has now also been used for intragastric titration of pH.
The measurement of intragastric pH with the Bravo capsule system is comparable to that of the gastric fistula model, and is useful for prolonged studies of gastric pH, even in free roaming conscious rats over days, as described. Although further studies are required, this study indicates the novel possibility for investigating the acid and gut hormone secretion under more physiological conditions.
| 2. | Brodie DA, Knapp PG. The mechanism of the inhibition of gastric secretion produced by esophageal ligation in the pylorus-ligated rat. Gastroenterology. 1966;50:787-795. |
| 3. | Hakanson R, Hedenbro J, Liedberg G, Sundler F, Vallgren S. Mechanisms of acid secretion after pylorus and oesophagus ligation in the rat. J Physiol. 1980;305:139-149. |
| 4. | Lane A, Ivy AC, Ivy EK. Response of the chronic gastric fistula rat to histamine. Am J Physiol. 1957;190:221-228. |
| 5. | Emas S, Nylander G, Wallin B. Comparison of the dose-response curves for acid output to pentagastrin determined by two techniques in chronic gastric fistula rats. Digestion. 1981;22:94-100. |
| 6. | Pascaud XB, Roger AR, Genton MJ. Comparison of the step-dose and single-dose acid response to histamine and pentagastrin in chronic gastric fistula rats. Digestion. 1977;16:57-68. |
| 7. | Blandizzi C, Colucci R, Carignani D, Natale G, Lazzeri G, Crema F, Del Tacca M. Role of peripheral GABAB receptors in the regulation of pepsinogen secretion in anaesthetized rats. Eur J Pharmacol. 1995;294:191-200. |
| 8. | Short GM, Wolfe MM, McGuigan JE. Pentagastrin-stimulated gastric acid secretion by the isolated perfused rat stomach. Life Sci. 1984;34:2515-2523. |
| 9. | Kleveland PM, Haugen SE, Sandvik S, Waldum HL. The effect of pentagastrin on the gastric secretion by the totally isolated vascularly perfused rat stomach. Scand J Gastroenterol. 1986;21:379-384. |
| 10. | Kleveland PM, Waldum HL, Larsson H. Gastric acid secretion in the totally isolated, vascularly perfused rat stomach. A selective muscarinic-1 agent does, whereas gastrin does not, augment maximal histamine-stimulated acid secretion. Scand J Gastroenterol. 1987;22:705-713. |
| 11. | Suzuki T, Yamaguchi T, Odaka T, Kobayashi M, Seza A, Kouzu T, Yokosuka O. Four-day continuous gastric pH monitoring following anti-acid secretory drug administration: cross-over test to assess the early effects. Aliment Pharmacol Ther. 2008;27:66-71. |
| 12. | Kwiatek MA, Pandolfino JE. The Bravo pH capsule system. Dig Liver Dis. 2008;40:156-160. |
| 13. | Hirano I. Review article: modern technology in the diagnosis of gastro-oesophageal reflux disease--Bilitec, intraluminal impedance and Bravo capsule pH monitoring. Aliment Pharmacol Ther. 2006;23 Suppl 1:12-24. |
| 14. | Bhat YM, McGrath KM, Bielefeldt K. Wireless esophageal pH monitoring: new technique means new questions. J Clin Gastroenterol. 2006;40:116-121. |
| 15. | Chen EP, Mahar Doan KM, Portelli S, Coatney R, Vaden V, Shi W. Gastric pH and gastric residence time in fasted and fed conscious cynomolgus monkeys using the Bravo pH system. Pharm Res. 2008;25:123-134. |
| 16. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. |
| 17. | Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135-160. |
| 18. | Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2007;23:595-601. |
| 19. | Levin F, Edholm T, Ehrstrom M, Wallin B, Schmidt PT, Kirchgessner AM, Hilsted LM, Hellstrom PM, Naslund E. Effect of peripherally administered ghrelin on gastric emptying and acid secretion in the rat. Regul Pept. 2005;131:59-65. |
| 20. | Kolivas S, Shulkes A. Regulation of expression of the receptors controlling gastric acidity. Regul Pept. 2004;121:1-9. |
| 21. | Lloyd KC, Raybould HE, Tache Y, Walsh JH. Role of gastrin, histamine, and acetylcholine in the gastric phase of acid secretion in anesthetized rats. Am J Physiol. 1992;262:G747-G755. |
| 22. | Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283-288. |
| 23. | Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23-32. |
| 24. | Bassil AK, Haglund Y, Brown J, Rudholm T, Hellstrom PM, Naslund E, Lee K, Sanger GJ. Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract. Br J Pharmacol. 2007;150:58-64. |
| 25. | Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227-240. |
| 26. | Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160-168. |
| 27. | Scarpignato C, Pelosini I, Di Mario F. Acid suppression therapy: where do we go from here? Dig Dis. 2006;24:11-46. |
| 28. | Hagiwara T, Mukaisho K, Ling ZQ, Sugihara H, Hattori T. Development of pancreatic acinar cell metaplasia after successful administration of omeprazole for 6 months in rats. Dig Dis Sci. 2007;52:1219-1224. |
| 29. | Schubert ML. Regulation of gastric acid secretion. Curr Opin Gastroenterol. 1999;15:457-462. |
| 30. | Hagiwara T, Mukaisho K, Ling ZQ, Sakano T, Sugihara H, Hattori T. Rebamipide contributes to reducing adverse effects of long-term administration of omeprazole in rats. Dig Dis Sci. 2007;52:988-994. |
| 31. | Ryberg B, Bishop AE, Bloom SR, Carlsson E, Hakanson R, Larsson H, Mattsson H, Polak JM, Sundler F. Omeprazole and ranitidine, antisecretagogues with different modes of action, are equally effective in causing hyperplasia of enterochromaffin-like cells in rat stomach. Regul Pept. 1989;25:235-246. |
Peer reviewers: Kyoichi Adachi, MD, Department of Gastroenterology and Hepatology, Shimane University, School of Medicine Shimane, 89-1 Enya-cho, Izumo-shi Shimane 693-8501, Japan; Satoshi Osawa, MD, First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan
S- Editor Xiao LL L- Editor Rippe RA E- Editor Lin YP