Published online Jul 7, 2008. doi: 10.3748/wjg.14.4054
Revised: April 9, 2008
Accepted: April 16, 2008
Published online: July 7, 2008
AIM: To assess the results of endoscopic mucosal resection with a ligation device (EMR-L) combined with three dimensional endoscopic ultrasonography (3D-EUS) using an ultrasonic probe for rectal carcinoids. In addition, diagnosis of the depth and size of lesions by EUS was evaluated.
METHODS: Between January 2003 and March 2007, 20 patients underwent EMR-L with 3D-EUS using an ultrasonic probe (group A). 3D-EUS was combined with EMR-L at the time of injection of sterile physiological saline into the submucosal layer. For comparison, 14 rectal carcinoids that had been treated by EMR-L without 3D-EUS between April 1998 and December 2002 were evaluated as historical controls (group B). EUS was conducted for all of the patients before treatment to evaluate tumor diameter and depth of invasion. The percentage of complete resection and the vertical resection margin were compared between the two groups.
RESULTS: The depth of invasion upon histopathological examination was in complete agreement with the pre-operative findings by EUS. The tumor diameter determined by EUS approximated that found in the tissue samples. There were no significant differences in the gender, tumor sites or tumor diameters between the two groups. The rate of complete resection for groups A and B was 100% and 71%, respectively (P < 0.05). The vertical resection margin of group A was longer than that of group B.
CONCLUSION: EMR-L is effective as an endoscopic treatment for rectal carcinoids. In combination with 3D-EUS, safe and complete resection is further assured.
- Citation: Abe T, Kakemura T, Fujinuma S, Maetani I. Successful outcomes of EMR-L with 3D-EUS for rectal carcinoids compared with historical controls. World J Gastroenterol 2008; 14(25): 4054-4058
- URL: https://www.wjgnet.com/1007-9327/full/v14/i25/4054.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4054
Recent progress in colonoscopy has facilitated the diagnosis of many rectal carcinoids at an early stage. Rectal carcinoids ≤ 1 cm in diameter and a depth of invasion to the submucosal layer are adequately treated by local excision[1–4].
Conventional snare polypectomy or endoscopic mucosal resection (EMR) often result in unsatisfactory complete resection of colonic carcinoids[14–6]. Although various methods, including EMR with a ligation device (EMR-L), have been developed, problems still remain. We developed a resection technique of EMR-L combined with three-dimensional endoscopic ultrasonography (3D-EUS) using an ultrasonic probe, to confirm accurate injection of saline into the submucosal layer beneath the tumor, for more assured resection. The current study was conducted to evaluate the efficacy of EMR-L in combination with 3D-EUS for endoscopic resection of rectal carcinoids, compared with EMR-L alone.
Between April 1998 and March 2007, 36 cases (36 lesions) of rectal carcinoid underwent colonoscopy, and their histopathological appearance was thoroughly examined at our institution. Following observation by conventional colonoscopy, EUS was performed to determine the depth of invasion, tumor size and possible metastasis to the surrounding lymph nodes. To rule out distant metastasis, the procedures were followed by abdominal ultrasonography or CT examination. Those with hepatic metastases, or a complication with severe ulcerative colitis (one case each), were treated surgically. The subjects of the current study were the remaining 34 patients with a tumor diameter ≤ 10 mm and a tumor depth that reached the submucosal layer, who underwent EMR-L.
Between January 2003 and March 2007, 20 patients underwent EMR-L combined with 3D-EUS using an ultrasonic probe (group A). For comparison, 14 patients with rectal carcinoids treated by EMR-L alone between April 1998 and December 2002 were included in this study as historical controls (group B).
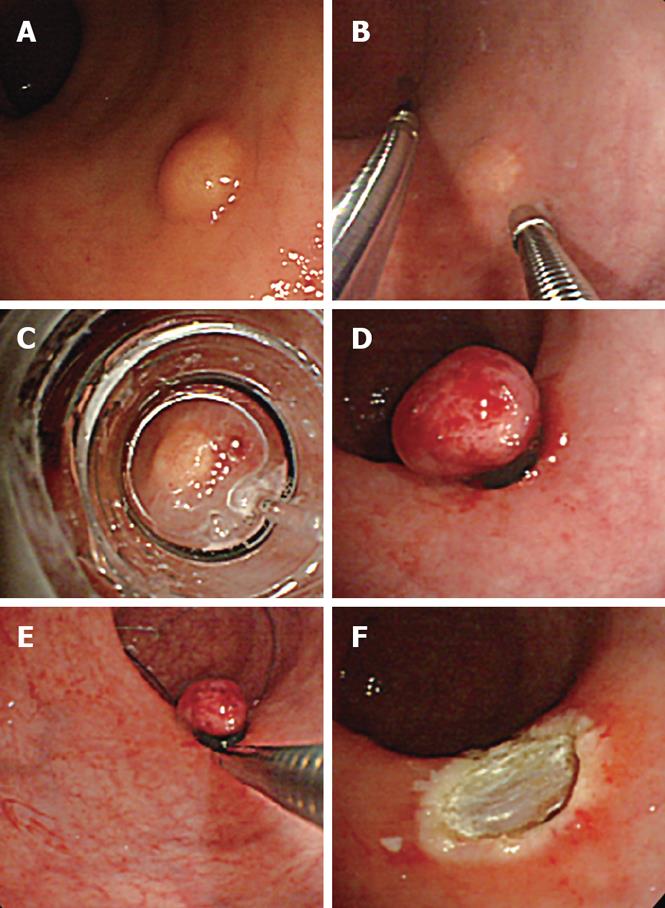
EMR-L, conducted according to Motohashi et al[7], was performed as follows: using a small-diameter colonoscope (CF-SV, Olympus. Tokyo Japan) with a ligation device for the treatment of esophageal varices, physiological saline was injected into the submucosal layer. After aspirating as much lesion as possible into the ligation device, the tumor was ligated with an elastic band. The section immediately below the elastic band was constricted by snare wire and resected using a high-frequency cutting current.
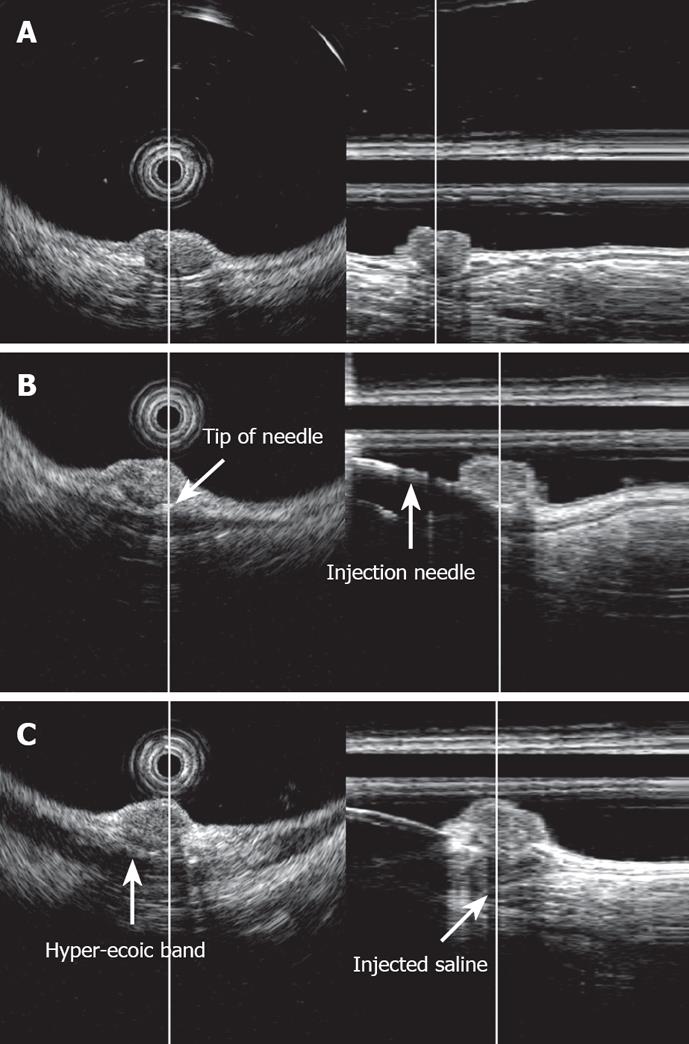
3D-EUS was combined with EMR-L at the time of injection of sterile physiological saline into the submucosal layer (Figure 1). Specifically, one channel of the two-channel colonoscope (CF-2T230, Olympus) was used for the saline injection, while the 3D ultrasonographic probe (UM-DP20-25R, 20 MHz; Olympus) was inserted through the other channel for scanning. At the time of saline injection, the tip of the injection needle was inserted while observing the ultrasonic radial and linear images, so that it would be located in the middle of the submucosal layer beneath the tumor. By recognizing the rise of the tumor caused by the injection and the adhesion of a hyper-echoic band directly beneath it, the tumor and its dissociation from the muscularis propria were confirmed (Figure 2). We used water-filling methods during EUS examination. For image processing, software for a three-dimensional image display (MAJ 1330, Olympus) was used. For observation of the images, dual plain reconstruction was employed. We compared tumor diameter and depth, determined by EUS, and the complete resection rate and vertical margin between the two groups.
All resected samples were stained with hematoxylin-eosin for histopathological diagnosis. When the tumor tissue was exposed at the deeper edge of the resected sample, or when evaluation of this section was not possible due to thermal degeneration, a judgment of “positive at the edge” was made. If adhesion of the normal submucosal layer was noted at the lower section of the tumor, it was classified as “negative at the edge”. The major axis was measured and recorded as the tumor diameter. The vertical margin was defined as the distance between the tumor and the edge of the specimen. We considered that the vertical margin of positive cases was 0 &mgr;m.
Following treatment, the patients were followed-up with colonoscopy, EUS and abdominal CT for 6 mo immediately following the treatment, and yearly thereafter.
For statistical analyses, the measured variables were expressed as mean ± SD. For group comparisons, the χ2 test, Fisher’s exact test and Mann-Whitney U test were conducted. A corrected P < 0.05 was considered to indicate statistical significance.
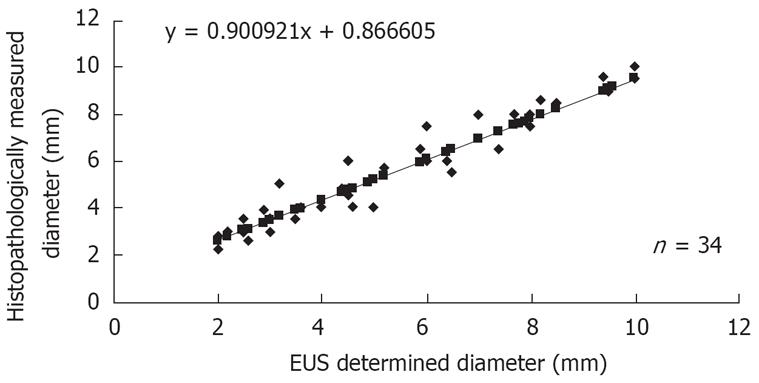
The EUS diagnosis before treatment determined that the tumor had reached deep into the submucosal layer in all 34 subjects. Histopathological examination also found tumor involvement in the submucosal layer in all cases, which demonstrated 100% diagnostic accuracy of EUS. A strong correlation was noted between EUS-determined and histopathologically measured tumor diameters (Figure 3). The two findings were very similar, with the difference between them being < 1 mm in 29 patients (85%) (Table 1).
| Difference in diameter (mm) | Cases (n = 34) |
| 0-0.5 | 20 |
| 1.0 | 9 |
| 1.5 | 4 |
| 2.0 | 1 |
When two groups were divided according to the method of treatment, there were no significant differences in age, gender or site of involvement and tumor diameter. Group B included four patients with “positive at the edge” and 10 with “negative at the edge,” with the rate of complete resection being 71%. All 20 subjects in group A were “negative at the edge” and the complete resection rate was significantly higher when compared with that in group B (Table 2). Mean tumor diameters between positive and negative at the edge in group B were 5.3 ± 1.7 mm and 6.7 ± 2.3 mm. Cases with incomplete resection were found in group B, regardless of tumor diameter. Mean vertical margin of group A was 1 231 ± 120.87 &mgr;m and that of group B was 634 ± 147.13 &mgr;m.
| Group A (n = 20) | Group B (n = 14) | P value | |
| Gender (M/F) | 11/9 | 6/8 | NS |
| Age (mean ± SD) | 58.7 ± 8.7 | 57.3 ± 11.3 | NS |
| Location (Rb/Ra) | 14/6 | 11/3 | NS |
| Tumor diameter, mm (mean ± SD) | 6.1 ± 2.3 | 5.9 ± 2.3 | NS |
| Complete resection rate, % (cut edge positive) | 100 (0) | 71.4 (4) | < 0.05 |
No procedure-related complications, such as hemorrhage and perforations, were found in either group.
Two patients had severe renal failure and another two did not agree to additional surgery. Therefore, the cases that were “positive at the edge” in group B underwent additional endoscopic treatment by conventional EMR and argon plasma coagulation. None of the additional resected specimens included tumor cells. No local recurrence or lymph node or hepatic metastases were noted in either group during a mean observation period of 48.7 mo.
The word carcinoid was first proposed by Oberndorfer in 1907[8]. Currently, carcinoids are characterized as slow-growing malignant neoplasms[9], which are epithelial tumors composed of endocrine cells with a unique histological pattern. In Japan, carcinoids of the digestive tract are frequently seen in the colorectal region, especially in the rectum, within 10 cm from the dentate line[10]. With the advancement and mounting popularity of colonoscopy, carcinoid of the digestive tract is being discovered more frequently.
In deciding on treatment for rectal carcinoids, the presence of metastases to lymph nodes or other organs is important. If the tumor is located in the muscularis propria, the rate of lymph node metastasis increases[11]. When the tumor diameter is ≤ 10 mm, the lesion has most often reached as far as the submucosal layer. For lesions with a diameter ≤ 10 mm, the metastatic rate is significantly lower than for those lesions with diameters ≥ 11 mm[12]. Therefore, for those lesions with a diameter of ≤ 10 mm and a depth that reaches the submucosal layer, local incision, especially endoscopic treatment, is frequently selected.
EUS is useful for measuring the diameter or depth of rectal carcinoids. Carcinoids are imaged by EUS as a low echoic region with a clear surrounding area[13]. It has been said that the tumor diameter determined by EUS rarely differs from the actual measurement[14]. The capacity of EUS to depict the intramural structure is outstanding, and its usefulness in determining tumor depth has already been widely recognized[313–16]. The accuracy of the pre-operative depth determination was 100% in the current study.
In endoscopic resection of rectal carcinoids, the complete resection rate for standard snare polypectomy or EMR is often low[4–6]. Therefore, various innovations have been made for complete resection. Resection using a two-channel scope[317], aspiration lumpectomy[18–20], and EMR-L have been utilized and their efficacy has been reported[72122]. Ono et al have applied EMR-L to fourteen patients[22] and Sakata et al[23] to eight, and all resulted in complete resection, which testifies to the efficacy of the method.
At our institution, prior to 1998, we resected rectal carcinoids by conventional polypectomy or using a two-channel scope, and EMR-L was adopted in 1998. We had initial success in complete resection in all cases[24], therefore, total complete resection rate has increased. But as the number of cases treated in this manner has increased, we have experienced a few cases with positive margin.
Aspiration lumpectomy can be used for resection from the deep submucosal layer[25]. By adding a ligation process with the aid of an elastic band, in theory, EMR-L should be able to reliably and safely resect the lesion and submucosal layer. Motohashi et al have stated that a sufficient quantity of saline injected into the submucosal layer results in safe ligation and resection[7]. Ono et al have reported that the vertical resection margin is greater using the EMR-L technique, however, there are still certain cases that exhibit positive margins regardless of the tumor diameter. It seems that these positive margins are due to technical factors. Moon et al have reported a procedure in which a snare device is left under the elastic band, so that the lesion can be ligated to include the deeper submucosal tissue and be resected more completely[26].
We believed that the accurate injection of saline into the submucosal layer beneath the tumor is necessary to elevate it upward, achieve effective ligation of the base of the lesion, and to resect the deep submucosal layer. Therefore, 3D-EUS was utilized to assist with the procedure at the time of the saline injection. While observing radial and linear ultrasonic images in real time, we objectively confirmed the insertion of a puncture needle into the middle section of the submucosal layer beneath the tumor, injection of physiological saline, elevation of the tumor, adhesion of a high-echoic band directly beneath the tumor, and dissociation from the muscularis propria. The lesion was constricted with a snare and resected. All the patients for whom EMR-L was combined with 3D-EUS exhibited negative margins, and compared with those without 3D-EUS, the rate of complete resection was significantly higher. This procedure tended to provide a deeper vertical resection margin.
There have been reports that confirm the dissociation of tumor from the muscularis propria by two-dimensional EUS (2D-EUS) during endoscopic treatment of submucosal tumors[2728]. The state of rectal carcinoid following a local injection may be confirmed by 2D-EUS[16]. When 2D-EUS is employed, the scope or probe must be moved while scanning to observe the entire lesion. Thus, a local saline injection to the submucosal layer cannot be conducted simultaneously with ultrasonic observation of the lesion. By employing 3D-EUS, on the other hand, both radial and linear images may be examined in real time while the injection is being administered. In this manner, the location of the tip of the local injection needle can be instantly confirmed or corrected. Furthermore, the state of the tumor being raised following the injection can be analyzed in three dimensions, which proves the efficacy of 3D-EUS.
Onozato et al have reported the use of endoscopic submucosal dissection (ESD) for rectal carcinoids[29]. However, the indication criteria for endoscopic treatment for rectal carcinoids included size ≤ 10 mm, and there is currently no significant difference between ESD and EMR-L for small rectal carcinoids in terms of complete resection rate. In the present study, the number of patients who underwent EMR-L with and without 3D-EUS was small, and the volume of injection was not compared between the two groups. This is a limitation of our retrospective study. Further studies are required to assess the volume of injection, long-term recurrence and patient survival. Also, the difference between ESD and EMR-L combined with 3D-EUS needs to be further evaluated.
EMR-L is significantly beneficial for endoscopic resection of rectal carcinoids. With the aid of 3D-EUS during the procedure, safe and complete resection is assured.
Recent progress in colonoscopic examination has facilitated the diagnosis of many rectal carcinoids at an early stage. Conventional snare polypectomy or EMR often yields unsatisfactory results in terms of complete resection of colonic carcinoids.
Various endoscopic treatments for colonic carcinoids were demonstrated.
EMR-L is as effective as endoscopic treatment for rectal carcinoids. In combination with 3D-EUS, safe and complete resection is assured.
Further studies are required to assess long-term recurrence and patient survival, and the difference between ESD and EMR-L combined with 3D-EUS needs to be further evaluated in a prospective study.
EMR-L is endoscopic mucosal resection with a ligation device. 3D-EUS is three-dimensional endoscopic ultrasonography.
This very interesting study assessed the results of EMR-L combined with 3D-EUS using an ultrasonic probe for rectal carcinoids. When comparing EMR-L with and without 3D-EUS, the volume of injection should be compared between the groups.
| 1. | Ishikawa H, Imanishi K, Otani T, Okuda S, Tatsuta M, Ishiguro S. Effectiveness of endoscopic treatment of carcinoid tumors of the rectum. Endoscopy. 1989;21:133-135. |
| 2. | Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy. 1997;29:171-175. |
| 3. | Kobayashi K, Katsumata T, Yoshizawa S, Sada M, Igarashi M, Saigenji K, Otani Y. Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum. 2005;48:285-291. |
| 4. | Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. Treatment of rectal carcinoid tumors. Am J Surg. 1990;160:262-265. |
| 5. | Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993;88:1949-1953. |
| 6. | Schindl M, Niederle B, Hafner M, Teleky B, Langle F, Kaserer K, Schofl R. Stage-dependent therapy of rectal carcinoid tumors. World J Surg. 1998;22:628-633; discussion 634. |
| 7. | Motohashi O, Sano H, Takagi S, Kiyohashi A, Saigenji K. The benefit of endoscopic mucosal resection of the rectal carcinoid tumor using a ligating device-experimental study and clinical use. Gastroenterol Endosc. 1997;39:1138-1143 (in Japanease with English abstract). |
| 8. | Oberndorfer S. Karzinoide Tumoren des Dunndarms. Frankfult Z Path. 1907;1:426-432. |
| 9. | Saha S, Hoda S, Godfrey R, Sutherland C, Raybon K. Carcinoid tumors of the gastrointestinal tract: a 44-year experience. South Med J. 1989;82:1501-1505. |
| 10. | Soga J. Carcinoid tumors: A statistical analysis of a Japanese series of 3126 reported and 1180 autopsy cases. Acuta Medica et Biologica. 1994;42:87-102. |
| 11. | Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112-119. |
| 12. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. |
| 13. | Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Mizutani K, Nakamura T. Carcinoid tumors of the gastroin-testinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1993;39:375-383. |
| 14. | Martinez-Ares D, Souto-Ruzo J, Varas Lorenzo MJ, Espinos Perez JC, Yaoez Lopez J, Abad Belando R, Alonso Aguirre PA, Miquel Colell JM, Vaquez Iglesias JL. Endoscopic ultrasound-assisted endoscopic resection of carcinoid tumors of the gastrointestinal tract. Rev Esp Enferm Dig. 2004;96:847-855. |
| 15. | Hurlstone DP, Cross SS, Sanders DS. 20-MHz high-frequency endoscopic ultrasound-assisted endoscopic mucosal resection for colorectal submucosal lesions: a prospective analysis. J Clin Gastroenterol. 2005;39:596-599. |
| 16. | Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44-49. |
| 17. | Iishi H, Tatsuta M, Yano H, Narahara H, Iseki K, Ishiguro S. More effective endoscopic resection with a two-channel colonoscope for carcinoid tumors of the rectum. Dis Colon Rectum. 1996;39:1438-1439. |
| 18. | Imada-Shirakata Y, Sakai M, Kajiyama T, Kin G, Inoue K, Torii A, Kishimoto H, Ueda S, Okuma M. Endoscopic resection of rectal carcinoid tumors using aspiration lumpectomy. Endoscopy. 1997;29:34-38. |
| 19. | Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, Matsumoto T, Kitano A, Arakawa T. Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res. 2000;28:241-246. |
| 20. | Nagai T, Torishima R, Nakashima H, Ookawara H, Uchida A, Kai S, Sato R, Murakami K, Fujioka T. Saline-assisted endoscopic resection of rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy. 2004;36:202-205. |
| 21. | Berkelhammer C, Jasper I, Kirvaitis E, Schreiber S, Hamilton J, Walloch J. "Band-snare" resection of small rectal carcinoid tumors. Gastrointest Endosc. 1999;50:582-585. |
| 22. | Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, Saito D. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc. 2003;57:583-587. |
| 23. | Sakata H, Iwakiri R, Ootani A, Tsunada S, Ogata S, Ootani H, Shimoda R, Yamaguchi K, Sakata Y, Amemori S. A pilot randomized control study to evaluate endoscopic resection using a ligation device for rectal carcinoid tumors. World J Gastroenterol. 2006;12:4026-4028. |
| 24. | Furuya M, Arai J, Ohta A, Satou K, Tada T, Hattori K, Ishizuka S, Kakemura T, Yoshida M, Yoshimoto K. Usefulness of Endoscopic resection using a ligation device for rectal carcinoids. Prog Dig Endosc. 1999;54:159 (in Japanease). |
| 25. | Kajiyama T, Hajiro K, Sakai M, Inoue K, Konishi Y, Takakuwa H, Ueda S, Okuma M. Endoscopic resection of gastrointestinal submucosal lesions: a comparison between strip biopsy and aspiration lumpectomy. Gastrointest Endosc. 1996;44:404-410. |
| 26. | Moon JH, Kim JH, Park CH, Jung JO, Shin WG, Kim JP, Kim KO, Hahn T, Yoo KS, Park SH. Endoscopic submucosal resection with double ligation technique for treatment of small rectal carcinoid tumors. Endoscopy. 2006;38:511-514. |
| 27. | Yokota T, Sugihara K, Yoshida S. Endoscopic mucosal resection for colorectal neoplastic lesions. Dis Colon Rectum. 1994;37:1108-1111. |
| 28. | Karita M, Tada M, Okita K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc. 1991;37:128-132. |