Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.4002
Revised: February 5, 2007
Accepted: April 7, 2007
Published online: August 7, 2007
AIM: To investigate the effect of endothelin-1 in the invasion of esophageal cancer and determine whether cathepsin B plays a role in the course.
METHODS: Western blotting was employed to detect the expression of ET-1 protein in 75 samples of esophageal squamous cell cancer and matched normal esophageal mucosa. Bosentan, a dual ET (A/B)-receptor antagonist, was used to inhibit the binding of endothelin-1 and its receptors and cut down its biological role. In vitro matrigel invasion assays were made to show the invasive ability of esophageal cancer cells with and without bosentan. Subsequently, we evaluated cathepsin B activity and expression in EC9706 cell with and without bosentan.
RESULTS: We found 74.7% (56/75) tumors had an overexpression of ET-1 protein by Western blotting. Bosentan significantly inhibited matrigel invasion of cancer cells in vitro. EC9706 cells have a positive expression of cathepsin B protein, and bosentan can down-regulate its expression and activity.
CONCLUSION: Endothelin-1 may enhance the invasive ability of human esophageal cancer cells, and its role is correlated with cathepsin B.
- Citation: Jiao WJ, Xu J, Pan H, Tian-You, Shen Y. Effect of endothelin-1 in esophageal squamous cell carcinoma invasion and its correlation with cathepsin B. World J Gastroenterol 2007; 13(29): 4002-4005
- URL: https://www.wjgnet.com/1007-9327/full/v13/i29/4002.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.4002
The epidemiology of esophageal cancer shows a striking geographic variation in the world. A 20% variation is observed between high-risk Chinese and low-risk western Africans[1]. Recently, advances in multiple therapies significantly improved the outcome of patients with esophageal squamous cell carcinoma (ESCC). However, the overall survival remains poor after curative treatment[2-4]. Actually, the development of new treatment protocols depends on improved knowledge of the molecular mechanisms controlling tumor invasion and metastasis.
Endothelin-1 (ET-1) is a small 21-residue peptide[5] which plays a biological role by binding two receptor subtypes, endothelin A and endothelin B receptors, belonging to the family of G-protein-linked receptors with seven transmembrane-spanning domains[6]. Recent studies[7-11] have suggested that endothelin-1 may play an important role in tumorigenesis, tumor progression and metastasis presumably by various mechanisms, including mitogenesis, inhibition of apoptosis and angiogenesis.
Cathepsin B is a kind of cysteine protease that actively participates in the proteolytic degradation of the extracellular matrix. It is believed that cathepsin B plays a critical role in tumor progression, and shows an over-expression in certain tumors of the lung, breast, stomach, colon, esophagus and prostate[12-14].
To our knowledge, little is known about the significance of ET-1 in the esophageal cancer invasion and metastases. In this study, we investigated the role of ET-1 in esophageal cancer invasion and its correlation with cathepsin B.
ESCC and adjacent almost normal tissues with size of about 0.5 cm3 were collected from 75 patients in Peking University First Hospital and Beijing Friendship Hospital. Fresh samples were stored in liquid nitrogen and each sample was confirmed by histological examination. Total proteins of the samples were extracted using Trizol solution (Life Technologies, Rochville, ML).
A human esophageal squamous cell carcinoma cell line EC9706 was maintained in our laboratory. All culture reagents were from Invitrogen. Cells were cultured in serum-free medium for 24 h before experiment.
Cells and tissues were harvested, rinsed twice with PBS, and protein preparation was performed according to the manufacturer’s instruction (Santa Cruz). The extracted proteins (20 μg) were subjected to 15 % polyacrylamide gel electrophoresis in the presence of SDS, and transferred onto PVDF membranes, and nonspecific binding was blocked with 5% nonfat dry milk for 1 h at room temperature. ET-1 and cathepsin B were detected by mouse monoclonal antibodies (Santa Cruz), and horseradish peroxidaselabeled anti-mouse immunoglobulin (Santa Cruz) was used as the secondary reagent. Detection was performed using an ECL system (Amersham-Pharmacia).
The chemoinvasion assay was done with a Boyden chamber and 8-μm pore size polyvinyl pyrrolidone-free polycarbonate nucleopore filters (Costar, USA) according to prior reference[15]. The filters were coated with an even layer of 0.5 g/L matrigel (Becton Dickinson, America). The lower compartment of the chamber was filled with 100 nmol/L ET-1 and/or 10 μmol/L bosentan (Bachem, Switzerland). Serum-starved EC9706 cells (5 × 105/mL) were placed in the upper compartment. After 24 h of incubation at 37°C, the filters were removed, air dried and stained. The invasion was quantified by counting cells in ten high power fields (20% of total filter area). Each experimental point was analyzed in triplicate.
The activity of cathepsin B was determined using the specific substrate Z-RR-AMC (Santa Cruz) according to the manufacturer’s instructions. Cell pellets of EC9706 were lysed in assay buffer (50 mmol/L acetate buffer, pH 5.5, 2.5 mmol/L DTT, 2.5 mmol/L EDTA) with 0.1% Triton X-100. The samples were centrifuged, and supernatants of cells were incubated with enzyme substrate for 24 h. The reaction was terminated, and stained cells were observed for cathepsin B activity under a fluorescence microscope. All quantifications were done in triplicate.
Western blotting was conducted to verify the differential expression of ET-1 in ESCC at protein level. Results in the above-mentioned 75 cases showed that ET-1 protein has a up-regulation in 74.7% (56/75) examined ESCC tissues relative to the matched normal tissues (Figure 1).
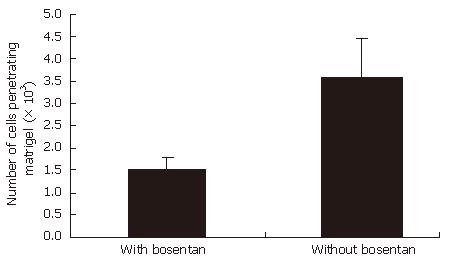
EC9706 cells were able to migrate through the matrigel-coated membrane. In our study, invasive ability remarkably decrease in EC9706 cells with bosentan, compared with the cells without bosentan (P < 0.001). Figure 2 shows the number of cells penetrating matrigel in the invasion assays in two groups.
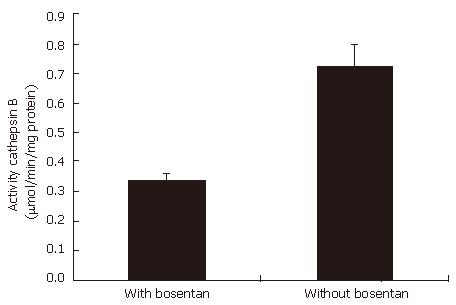
Western blotting shows that bosentan down-regulates cathepsin B expressions in EC9706 cells, compared with the group without bosentan. Figure 3 shows the expressions cathepsin B protein detected by Western blotting in the cells with and without bosentan. Cathepsin B was found to be 2-fold lesser active in group with bosentan than in the group without bosentan. Figure 4 shows the activity of cathepsin B in the supernatants of these cells.
It is well established that ET-1 may act as an autocrine or paracrine growth factor mediating through its receptor, ETAR or ETBR. Some findings[16-19] suggest that endothelin axis plays an important role in a series of events related to tumor development, including mitogenesis[20,21] and escape from apoptosis[22,23]. Moreover, ET-1 expression has been linked to induction of endothelial cell growth, angiogenesis[24-26] and epithelial to mesenchymal transition[27-29], resulting in an increased invasiveness and metastases of some tumors. Our results showed endothelin-1 protein has a high expression in most esophageal cancer tissues. So we suppose that endothelin-1 may play a role in esophageal tumor development.
One of the features of primary malignant esophageal tumors is their ability to invade locally into normal tissues. In order to study the effect of ET-1 in esophageal cancer invasion, we used a dual pharmacologic antagonist of endothelin receptor, bosentan, to test whether the interference with ET/ET (A, B) R autocrine loop would affect invasion. Bosentan is a specific competitive dual endothelin receptor antagonist of low molecular weight. It inhibits ET-1 action through combination with both ETA and ETB receptors. In our invasion assays, cellular invasion was decreased by adding bosentan to the esophageal tumor cells, compared to the control group. It suggests that endogenous ET-1 acts as an autocrine modulator and its expression has a close correlation with invasion of esopohageal cancer cells.
Tumor invasion and metastasis is a multi-step process that involves, at various stages, the penetration of host extracellular matrix by cancer cells. The ability of tumor cells to invade tissues and metastasize is thought to involve an increased expression of proteinases and/or a decrease in the levels of proteinase inhibitors[30]. Penetration and degradation of extracellular matrix elements of metastasizing tumor cells are key steps in the metastatic cascade of cancer cells[31]. ET-1, acting through ETAR or ETBR, consistently induces the activity of multiple metastasis-related proteinases, such as matrix metalloproteinases (MMP) and the urokinase-type plasminogen activator system (uPA)[15], and mediates extracellular matrix degradation. However, we have not fully understood the correlations among ET-1, proteinases (for example, cathepsin B) and invasion of esophageal cancer.
Cathepsin B is the major representative of the cysteine proteinases and is present in lysosomes of all types of cells under normal conditions. Cathepsin B is also engaged in proteolytic cascades to activate other proteinases, which in turn, mediate extracellular matrix degradation[32]. Localized degradation of the extracellular matrix by proteases such as cathepsin B is a necessary step in tumor invasion[13,14]. Lin and Hughes reported that cathepsin B had an overexpression in esophageal adenocarcinoma[33,34]. In our study, bosentan can remarkably down-regulate cathepsin B activity and expression in esophageal cancer cell line EC9706. The phenomenon suggests that, via up-regulating cathepsin B, ET-1 may play an important role in promoting esophageal squamous cancer cell invasion.
In conclusion, endothelin-1 may enhance the invasive ability of human esophageal cancer cells, and the role of endothelin-1 is correlated with cathepsin B.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 2. | Portale G, Hagen JA, Peters JH, Chan LS, DeMeester SR, Gandamihardja TA, DeMeester TR. Modern 5-year survival of resectable esophageal adenocarcinoma: single institution experience with 263 patients. J Am Coll Surg. 2006;202:588-596; discussion 596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2232] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 4. | Shiozaki H, Doki Y, Kawanishi K, Shamma A, Yano M, Inoue M, Monden M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery. 2000;127:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86:2863-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1654] [Cited by in RCA: 1637] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 6. | Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Wülfing P, Kersting C, Tio J, Fischer RJ, Wülfing C, Poremba C, Diallo R, Böcker W, Kiesel L. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10:2393-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Spinella F, Rosanò L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850-27855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Salani D, Taraboletti G, Rosanò L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosanò L, Trisciuoglio D, Spinella F, Bagnato A. Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol Pharmacol. 2002;61:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Boldrini L, Gisfredi S, Ursino S, Faviana P, Lucchi M, Melfi F, Mussi A, Basolo F, Fontanini G. Expression of endothelin-1 is related to poor prognosis in non-small cell lung carcinoma. Eur J Cancer. 2005;41:2828-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Sloane BF. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990;1:137-152. [PubMed] |
| 13. | Krueger S, Kalinski T, Wolf H, Kellner U, Roessner A. Interactions between human colon carcinoma cells, fibroblasts and monocytic cells in coculture--regulation of cathepsin B expression and invasiveness. Cancer Lett. 2005;223:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Yang ZQ, Imoto I, Fukuda Y, Pimkhaokham A, Shimada Y, Imamura M, Sugano S, Nakamura Y, Inazawa J. Identification of a novel gene, GASC1, within an amplicon at 9p23-24 frequently detected in esophageal cancer cell lines. Cancer Res. 2000;60:4735-4739. [PubMed] |
| 15. | Rosanò L, Varmi M, Salani D, Di Castro V, Spinella F, Natali PG, Bagnato A. Endothelin-1 induces tumor proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res. 2001;61:8340-8346. [PubMed] |
| 16. | Donckier JE, Michel L, Van Beneden R, Delos M, Havaux X. Increased expression of endothelin-1 and its mitogenic receptor ETA in human papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2003;59:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Venuti A, Salani D, Manni V, Poggiali F, Bagnato A. Expression of endothelin 1 and endothelin A receptor in HPV-associated cervical carcinoma: new potential targets for anticancer therapy. FASEB J. 2000;14:2277-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wülfing P, Tio J, Kersting C, Sonntag B, Buerger H, Wülfing C, Euler U, Boecker W, Tulusan AH, Kiesel L. Expression of endothelin-A-receptor predicts unfavourable response to neoadjuvant chemotherapy in locally advanced breast cancer. Br J Cancer. 2004;91:434-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Mai HQ, Zeng ZY, Zhang HZ, Hou JH, Mo HY, Guo X, Min HQ, Hong MH. Correlation of endothelin A receptor expression to prognosis of nasopharyngeal carcinoma. Ai Zheng. 2005;24:611-615. [PubMed] |
| 20. | Kawanabe Y, Hashimoto N, Masaki T. Effects of extracellular Ca2+ influx on endothelin-1-induced intracellular mitogenic cascades in C6 glioma cells. Eur J Pharmacol. 2002;435:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Kawanabe Y, Hashimoto N, Masaki T. B103 neuroblastoma cells predominantly express endothelin ET(B) receptor; effects of extracellular Ca(2+) influx on endothelin-1-induced mitogenesis. Eur J Pharmacol. 2001;425:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Nelson JB, Udan MS, Guruli G, Pflug BR. Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia. 2005;7:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Duan J, Dai S, Fang CX, Sun R, Shavali S, Sharma SK, Ebadi M, Ren J. Phytoestrogen alpha-zearalanol antagonizes homocysteine-induced imbalance of nitric oxide/endothelin-1 and apoptosis in human umbilical vein endothelial cells. Cell Biochem Biophys. 2006;45:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Bhargava S, Stummeyer T, Hotz B, Hines OJ, Reber HA, Buhr HJ, Hotz HG. Selective inhibition of endothelin receptor A as an anti-angiogenic and anti-proliferative strategy for human pancreatic cancer. J Gastrointest Surg. 2005;9:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Medinger M, Adler CP, Schmidt-Gersbach C, Soltau J, Droll A, Unger C, Drevs J. Angiogenesis and the ET-1/ETA receptor system: immunohistochemical expression analysis in bone metastases from patients with different primary tumors. Angiogenesis. 2003;6:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Knowles J, Loizidou M, Taylor I. Endothelin-1 and angiogenesis in cancer. Curr Vasc Pharmacol. 2005;3:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, Leclerc S, Moreau A, Moldovan F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond). 2006;110:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Rosanò L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649-11657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Hagemann T, Binder C, Binder L, Pukrop T, Trümper L, Grimshaw MJ. Expression of endothelins and their receptors promotes an invasive phenotype of breast tumor cells but is insufficient to induce invasion in benign cells. DNA Cell Biol. 2005;24:766-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s-5059s. [PubMed] |
| 31. | Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161-195. [PubMed] |
| 32. | Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066-13076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 450] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 33. | Lin L, Aggarwal S, Glover TW, Orringer MB, Hanash S, Beer DG. A minimal critical region of the 8p22-23 amplicon in esophageal adenocarcinomas defined using sequence tagged site-amplification mapping and quantitative polymerase chain reaction includes the GATA-4 gene. Cancer Res. 2000;60:1341-1347. [PubMed] |
| 34. | Hughes SJ, Glover TW, Zhu XX, Kuick R, Thoraval D, Orringer MB, Beer DG, Hanash S. A novel amplicon at 8p22-23 results in overexpression of cathepsin B in esophageal adenocarcinoma. Proc Natl Acad Sci USA. 1998;95:12410-12415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Ma JY E- Editor Lu W