Published online May 28, 2007. doi: 10.3748/wjg.v13.i20.2858
Revised: February 25, 2007
Accepted: March 1, 2007
Published online: May 28, 2007
AIM: To reserve the rare Chinese familial adenomas polyp (FAP) family resource and to investigate the clinical features of FAP in Chinese for its diagnosis.
METHODS: Clinical features of patients with FAP were investigated. If there is any question, their medical records were verified. Blood sample was taken and lymphocyte immortal cell lines were established with modified EB-transformation methods. Congenital hypertrophy of retinal pigment epithelium (CHRPE) was checked by an experienced ophthalmologist.
RESULTS: Twenty seven families including 21 classical FAP (CFAP) families, 3 attenuated FAP (AFAP) families, and 3 suspected AFAP families were investigated. A total of 116 lymphocyte immortal cell lines were established from 26 families. In all the FAP families, colorectal cancer occurred at the mean age of 42.84 years. Of the 16 families checked, 15 (93.75%) had CHRPE. The mean number of patients suffering from colorectal neoplasm was 3.14 in CFAP families and 2.0 in AFAP families (P < 0.01). The mean oldest age at diagnosis of FAP was 41.75 years in CFAP families, and 58.67 years in AFAP families, respectively (P < 0.01). Mean age of development of colorectal cancer was 42.23 in CFAP and 57.33 years old in AFAP (P < 0.01). Mean of the earliest age at diagnosis of FAP was 29.95 years in the FAP families with a positive family history and 46.80 years in the FAP families with a negative family history (P < 0.01). The ratio of extra-intestinal tumors to colorectal neoplasms was different in the two kinds of families with positive and negative family history (P < 0.01).
CONCLUSION: Additional use of ciclosporin will effectively improve to establish lymphocyte immortal cell lines with modified EB- transformation methods. In Chinese FAP, there was a high frequency of CHRPE , and a later age at diagnosis and a later age of development of colorectal cancer in AFAP. And earlier age at diagnosis in FAP with positive family history was also found that will help to diagnose various kinds of FAP in Chinese.
- Citation: Cai SR, Zhang SZ, Zheng S. Clinical features of familial adenomas polyps in Chinese and establishment of its immortal lymphocyte cell lines. World J Gastroenterol 2007; 13(20): 2858-2861
- URL: https://www.wjgnet.com/1007-9327/full/v13/i20/2858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i20.2858
Familial adenomatous polyposis (FAP) is one of the two commonest familial syndromes that predispose to colorectal cancer in China. Classically, FAP is characterized by the occurrence of hundreds to thousands of colorectal polyps, which give rise to colorectal cancer if left untreated. The disease is caused by mutations in the adenomatous polyposis coli (APC) tumor suppressor gene. Approximately 50% of offsprings in FAP family will be affected, and prophylactic surgical intervention is fundamental to avoid the development of colorectal cancer[1]. Classical FAP is defined clinically by the finding of at least 100 colorectal adenomas polyps. Another different kind of FAP named attenuated FAP (AFAP) in which adenomas are less than one hundreds, and the average age of cancer onset is also older (55 years old) than that of classical FAP (39 years old)[2,3]. However, little is known about the clinical manifestations of these two kinds of FAP in Chinese. It is also of vital importance to collect and reserve FAP family resource and to analyze FAP’s phenotype that will extend our understanding of FAP and its diagnosis in Chinese.
All FAP families were collected from the People’s Republic of China, mainly from Zhejiang Province (24 families). Classical FAP (CFAP) is defined clinically by the finding of at least 100 colorectal adenoma polyps. In attenuated FAP (AFAP), less than 100 adenomas could be found at the age of over 40 years, and at least one of the following has a family history of FAP or colorectal cancer, congenital hypertrophy of retinal pigment epithelium (CHRPE). If a proband was found in less than 100 adenomas and the age was less than 40 years, this family was defined as suspected AFAP. The clinical features of patients in one FAP family were investigated. If there is any question, medical records in hospital were verified. Blood samples were taken to establish immortal lymphocyte cell lines with informed consent.
Lymphocyte separation and cultivation: About 5 mL blood was drawn into two BD vacutainers containing 3.6 mg k2 EDTA, and 2-2.5 mL blood was doubly diluted with wash media such as RPMI 1640 culture, and added into one tube containing Ficoll-plaque, then centrifuged at 2400 r/min for 13 min. The lymphocytes at the interface were collected and washed with RPMI 1640.
EB virus transformation (immortalization)[4,5]: B95-8 cell line producing Epstein-barr virus (EBV) was cultured and 1000 mL cell mixture was centrifuged at 2000 r/min for 5 min at 4°C. The supernatants were collected and centrifuged at 10 000 r/min for 2 h at 4°C. After the supernatants were discarded, EBV was stayed in the tube and resolved in 10 mL RPMI 1640 culture through a 0.45 μm filter and stored at -80°C. Lymphocytes after separation were resolved in an initiation medium (RPMI 1640, + 10%-15% heat-inactivated fetal bovine serum, + 2 μg/mL ciclosporin) in 24-well plates (Falcon), and 50 μL EB virus suspension was added. The lymphocytes were incubated at 37°C in an atmosphere containing 5% CO2 for one week without changing the medium. Then, the medium was replaced with about half of the total volume of the initiation medium every 2-3 d. After 10-15 d, clones apppeared and grew rapidly. If the cell density was over 5 × 105, they transfomed into another 25 mL plate and cultured for 10-15 d and freezed in liquid nitrogen.
Student's t test was used to analyze the mean between different kinds of FAP, and chi-square test was used to compare the ratio of extra-intestinal tumors and colorectal neoplasms with SPSS 10.0 software.
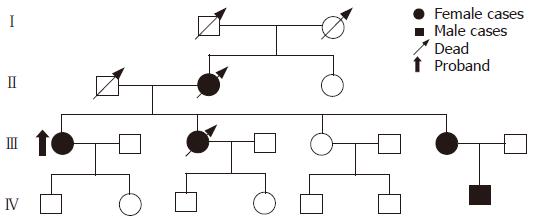
Twenty-seven FAP families were investigated. Pedigrees such as classical FAP were plotted (Figure 1).
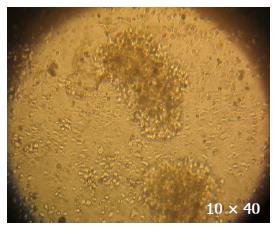
In the 27 families, one family refused to afford blood samples although its members received investigation. One hundred and thirty-two blood samples were taken from 26 FAP families and 125 blood samples were used to establish EB-transformed cell lines, in which 116 lymphocyte immortal cell lines were successfully established (Figure 2) and 9 relatives’ lymphocytes failed to set up immortal cell lines due to epiphyte contamination. The rate of successfully transformed cell lines was 92.8% (116/125).
In the 27 families, there were 80 cases of colorectal neoplasm (including adenoma and cancer). Among them, colorectal cancer was diagnosed in 29 members from 19 FAP families at the age of 19-67 years, and colorectal cancer occurred at the mean age of 42.84 years. Six families were diagnosed with AFAP and suspected AFAP in which the number of polyps in probands was less than 100. Of the 6 families, 5 had 8 persons suffering from extra colonic cancer including 3 lung cancers, 2 live cancers, 1 vocal, stomach and small intestine cancer, respectively.
The phenotype in the three different FAP families is listed in Table 1 and Table 2, and the phenotype in FAP families with positive and negative family history is listed in Table 3 and Table 4.
| Kinds of FAP families | Number of families | Families withpositivefamily history | Mean number ofcolorectal neoplasmsb | Mean number ofcolorectal cancer | Mean of the earliest age atdiagnosis in one family |
| CFAP | 21 | 16 | 3.14 ± 1.59 | 1.29 ± 1.10 | 32.9 ± 10.31 |
| AFAP | 3 | 3 | 2.00 ± 0.00 | 1.50 ± 0.71 | 42.0 ± 14.0 |
| Suspected AFAP | 3 | 3 | 2.67 ± 0.58 | 0.67 ± 0.58 | 25.0 ± 3.61 |
| Kinds of FAP families | Mean of the latest age atdiagnosis in one familyb | Mean age ofsymptom appearing | Mean age of colorectalcancer developmentb | Extra coloniccancers/colorectal neoplasms | Positive CHRPEfamilies/total checked families |
| CFAP | 41.75 ± 7.75 | 26.56 ± 12.36 | 42.23 ± 9.24 | 6/66 | 11/12 |
| AFAP | 58.67 ± 7.37 | 37.50 ± 21.92 | 57.33 ± 8.39 | 2/6 | 2/2 |
| Suspected AFAP | 41.6 ± 10.02 | 18.00 ± 4.24 | 33.67 ± 13.58 | 0 | 2/2 |
| Status offamily history | Mean of the latest age atdiagnosis in one family | Mean age ofsymptom appearing | Mean age of colorectalcancer development | Extra coloniccancers/colorectal neoplasmsb | Positive CHRPE families/totalchecked families |
| Positive | 44.05 ± 9.64 | 25.64 ± 12.36 | 43.15 ± 11.48 | 5/75 | 12/13 |
| Negaitive | 33.33 ± 17.01 | 3/5 | 3/3 |
There was a difference in the number of colorectal neoplasms (due to their definitions) between CFAP and AFAP families and the mean oldest age at diagnosis of FAP and colorectal cancer. The mean oldest age at diagnosis of FAP and the mean age of colorectal cancer development in AFAP families were later than those in CFAP families.
There was a difference in the mean number of colorectal neoplasms (due to classification), the mean youngest age at diagnosis of FAP and the ratio of extra-intestinal tumors to colorectal neoplasms in the FAP families with a different family history. In the FAP families with a positive history, onset of FAP was 15 years earlier than which in the FAP families with a negative family history.
In Western countries, there is a national or international collection net of FAP. In China, no national net for collection of FAP has been established. However, more attention is paid to the collection and reservation of the rare resource of hereditary cancer families such as FAP and hereditary nonpolyposis colorectal cancer families than before. We have established 116 lymphocyte immortal cell lines (92.8%) with modified EB-transformation method for the reservation of FAP family resource. Additional use of 2 μg/mL ciclosporin can effectively prevent T lymphocytes to attack B lymphocytes.
The phenotypic variability in patients with FAP has been recognized for many years[6]. However, it is uncertain whether FAP clinical manifestations are different in Chinese and Western individuals. It was reported that colorectal cancer occurs at the age of 39 years in Western populations[3] and in Chinese at the mean age of 42.23 years. It was also reported that, in AFAP families, colorectal cancer occurs at the age of 55 years in Western populations[2] and at the age of 57.33 years in Chinese. In Chinese, Symptoms of colorectal cancer appear 10 years later in AFAP families than in CFAP families, while symptoms of colorectal cancer appear in Western population at the age of 33 years[5]. In addition, some items are different in the two kinds of FAP families in Chinese. In the present study, the mean number of colorectal neoplasms was higher in CFAP families (3.14) than in AFAP families (2.0, P < 0.01), the mean oldest age at diagnosis of FAP in one family was later in AFAP than in CFAP (P <0.01). All these findings may further improve our ability to predict AFAP and CFAP. Based on these data, it may be concluded that the development time of colorectal cancer may be later in Chinese than which in Western populations. To confirm these hypotheses may need a larger sample of Chinese FAP families.
In Chinese FAP families, the mean youngest age at diagnosis of FAP in one family was 29.95 and 46.8 years, respectively in the two groups with positive (22 families) and negative (5 families) family history (P < 0.01), indicating that a positive FAP family history is a high-risk factor for developing colorectal cancer at a young age. The ratio of extra-intestinal tumors to colorectal neoplasms was 5/75 and 3/5 in the two kinds of families with a different family history (P < 0.01).
CHRPE is a common symptom of FAP. It was reported that there is no association between CHRPE characteristics and FAP phenotype variants[7]. It was reported that the rate of CHRPE is 75%-80% in Western FAP patients[8] and 93.75% in Chinese FAP families. CHRPE is more frequent in Chinese FAP families. In the present study, 8 asymptomatic family members with CHRPE were diagnosed with FAP, indicating that CHRPE is a good marker for the diagnosis of FAP in Chinese.
Clinicians must be aware of the broad range of variable clinical features of FAP due to its different variants. In China, it is of vital importance to establish a national net to collect FAP families and to analyze its phenotype characteristics and its association with genotype.
| 1. | Lynch HT, Smyrk TC. Classification of familial adenomatous polyposis: a diagnostic nightmare. Am J Hum Genet. 1998;62:1288-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Spirio L, Olschwang S, Groden J, Robertson M, Samowitz W, Joslyn G, Gelbert L, Thliveris A, Carlson M, Otterud B. Alleles of the APC gene: an attenuated form of familial polyposis. Cell. 1993;75:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 369] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Lynch HT, Smyrk T, McGinn T, Lanspa S, Cavalieri J, Lynch J, Slominski-Castor S, Cayouette MC, Priluck I, Luce MC. Attenuated familial adenomatous polyposis (AFAP). A phenotypically and genotypically distinctive variant of FAP. Cancer. 1995;76:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA. 1973;70:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 688] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Sugimoto M, Tahara H, Ide T, Furuichi Y. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res. 2004;64:3361-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Howe JR, Guillem JG. The genetics of colorectal cancer. Surg Clin North Am. 1997;77:175-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Touriño R, Conde-Freire R, Cabezas-Agrícola JM, Rodríguez-Aves T, López-Valladares MJ, Otero-Cepeda JL, Capeans C. Value of the congenital hypertrophy of the retinal pigment epithelium in the diagnosis of familial adenomatous polyposis. Int Ophthalmol. 2004;25:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours (Pathology and Gennetics of Tumors of the Digestive System). Lyon (France): International Agency for Research on Cancer Press 2000; 120-125. |
S- Editor Zhu LH L- Editor Wang XL E- Editor Lu W