Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2381
Revised: December 20, 2006
Accepted: January 25, 2007
Published online: April 28, 2007
Mucocoele of the appendix occurs when obstruction of the appendiceal lumen results in mucus accumulation and consequent abnormal dilatation. The most impor-tant aetiology, from a surgical perspective, is either mucinous cystadenoma or cystadenocarcinoma. In the latter, a spontaneous or iatrogenic rupture of the mucocoele can lead to mucinous intraperitoneal ascites, a syndrome known as pseudomyxoma peritonei. Optimal management of mucoceles is achieved through accurate preoperative identification and subsequent careful resection. We report two cases and subsequently discuss the clinical presentation of mucocoeles, their association with pseudomyxoma peritonei and an optimal management of both conditions.
- Citation: Dixit A, Robertson JH, Mudan SS, Akle C. Appendiceal mucocoeles and pseudomyxoma peritonei. World J Gastroenterol 2007; 13(16): 2381-2384
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2381
Mucocoele is a rare condition of the appendix, charac-terised by a cystic dilation of the lumen due to obstruction and consequent accumulation of mucus. The reported incidence is between 0.2% and 0.3%[1]. The condition is more common in females (4:1) and in people older than 50 years of age[2]. Aetiological factors range from inflammatory to neoplastic and the treatment is empirically directed towards the underlying pathology. Appropriate management of neoplastic mucoceles is important to prevent the development of mucinous intraperitoneal ascites resulting in a syndrome called pseudomyxoma peritonei, commonly referred to as ‘jelly belly’[3] and constitutes approximately 1% of all colorectal cancers in the USA[4]. We describe two cases, the first an appendiceal mucocoele, and the second a case of pseudomyxoma peritonei, both of which illustrate the problem and finally we discuss their management.
A 66-year-old gentleman presented with recurrent attacks of left sided abdominal pain (mimicking classical diverticulitis) over a period of twenty years. Constipation seemed to be the main stimulus and the attacks settled down with antibiotics and occasional laxatives. On this occasion he presented with an acute exacerbation of pain and nausea. His past history included hermaphroditism with removal of primordial ovaries in childhood. Computed tomography (CT) of the abdomen and pelvis revealed a 4-centimeter, well-defined mucocoele of the appendix. Colonoscopy showed inactive diverticular disease in the sigmoid colon and a 4-millimeter fronded polyp in the right colon close to the caecum. The caecum was distorted because of the mass in the appendix and no attempt was made to intubate it for fear of rupturing it.
Laparoscopic assisted right hemicolectomy was perfor-med. Extreme care was taken to ensure the tumour was not handled directly by laparoscopic instruments. There was no sign of intra-abdominal fluid or lymphadenopathy.
Histology revealed gross appendiceal distension (measuring 8.5 cm × 2.5 cm) with associated mucus. The appendix base opened into a hemispherical chamber distended with mucin (Figure 1). Histo-pathological analysis showed a mucinous cystadenoma. Five lymph nodes examined were free from the tumour. There was low potential for local recurrence or pseudomyxoma peritonei. The patient was discharged on the fourth post-operative day after an uneventful recovery and remained asymp-tomatic in the following 9 mo.
The second case depicts a more unfortunate patient presenting with established pseudomyxoma peritonei. A 57-year-old gentleman initially presented in a middle eastern county with abdominal distension, lower limb oedema and weight loss. He had a history of hypertension, diabetes and hepatitis C with reported associated cirrhosis. Despite normal blood results and an inconclusive peritoneal aspiration, an abdominal CT scan suggested pseudomyxoma. A diagnostic laparoscopy showed a large amount of mucinous material and obtained samples confirmed the diagnosis of pseudomyxoma.
At laparotomy, 8-10 kg of a gelatinous tumour was removed from his abdomen. Histo-pathological analysis reconfirmed the diagnosis of pseudomyxoma peritonei. Treatment with oral Tegafur-uracil (UFT) ensured a symptom free status for six months. Subsequently he developed abdominal distension, constipation and acid regurgitation followed by left sided abdominal pain and night sweats, and finally he was referred to our clinic.
We performed an abdominal CT scan, which showed bulky abdominal disease with associated mediastinal spread. In the superior mediastinum, a 3 cm mass was displacing the trachea, with disease extending into the retrocardiac region. Serumalbumin and haemoglobin were low, and tumour markers including CEA, Ca125 and CA19.9 were borderline elevated.
A decision for surgical debulking before administration of systemic therapy was made. Pre-operatively, an ultra-sound guided liver biopsy was undertaken to deter-mine the extent of his cirrhosis. The scan showed a large com-plex mass occupying the upper abdomen. The ultrasound guided hepatic biopsy showed normal architecture but surprisingly revealed amyloidosis. Pulmonary function tests were satisfactory. Once optimised, the patient under-went laparotomy. The findings included free mucin in the pelvis and a small bowel studded with tumour deposits. Moreover, the colon and stomach were encased as a single mass.
Histology of peritoneum revealed tissue extensively replaced by a deposit of mucinous adenocarcinoma. Intestinal type malignant cells lining the mucin-filled spaces were also seen.
Despite the administration of several cycles of systemic oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX 4TM), a CT scan six months post surgery showed considerable pseudomyxoma peritonei below the diaphragm. The right paratracheal and retrocardial masses were seen in the mediastinum and had no alteration in size.
Mucocoele of the appendix was recognized as a pathologic entity by Rokitansky in 1842 and was formally named by Feren in 1876[5]. Appendiceal mucoceles are uncommon entities arising from a variety of different pathologic processes, of which only a small subset are associated with development of pseudomyxoma peritonei. The pathological diagnosis determines further management[6].
There are four pathological entities described according to the characteristics of the epithelium[2,3,6,7]. (1) Simple or retention mucocoeles result from obstruction of the appendiceal outflow. This is usually by a faecolith and characterized by normal epithelium and mild luminal dilatation up to 1 cm. (2) Mucoceles with hyperplastic epithelium occur where luminal dilatation is also mild. These constitute 5%-25% of mucocoeles. (3) Mucinous adenoma/cystadenoma is the most common form, accounting for 63%-84% of cases. These exhibit mostly epithelial villous adenomatous changes with some degree of epithelial atypia. There is marked distention of the lumen up to 6 cm. (4) Malignant mucinous cystadenocarcinomas, represent 11%-20% of cases. These demonstrate glandular stromal invasion and/or presence of epithelial cells in the peritoneal implants. The luminal distention is usually severe.
Perforation results in dissemination of mucoid material in the peritoneal cavity. This material may be acellular or contain cells with varying degrees of dysplasia. Unlike colorectal cancer, they are usually less aggressive and rarely present with lymph node or liver metastasis[8]. Instead these cells spread to the peritoneal surfaces with a propensity to spare mobile portions of small bowel. Dispersion and establishment of cystadenocarcinoma cells throughout the abdomen lead to pseudomyxoma peritonei[1,9,10].
The clinical presentation of a mucocoele is varied and usually nonspecific. Only 50% of patients are symptomatic and it is an incidental finding in 50% at the time of surgery[2,5,6,11]. In the patients who are symptomatic, abdominal pain is most common followed by abdominal mass, weight loss, nausea, vomiting and acute appendicitis in decreasing order of frequency[11-13]. Rupture of a neo-plastic appendiceal mucocele may present as appendicitis[14].
On the other hand, pseudomyxoma peritonei is associa-ted with even more nonspecific symptoms. The most frequent presentation is gradually increasing abdominal circumference. Men may present with new onset hernia, while women can develop an ovarian mass[15].
Diagnosis of a mucocele is established either by abdo-minal CT scan or by ultrasound examination. CT scan appearances of a mucocoele include a characteristic, well encapsulated, round, thin walled cystic mass. Calcification is seen in 50% of cases[12,16]. Enhancing nodules in the mucocele wall are likely to suggest cystadenocarcinoma[17]. It is important to note that mucocoeles less than 2 cm are rarely malignant[11].
Larger mucocoeles (6 cm or more) are associated with cystadenoma or cystadenicarcinoma and a higher perforation rate (20%)[18,19].
Ultrasonography usually shows a cystic, encapsulated lesion, firmly attached to the caecum. Internal variable echogenicity is related to the density of mucus. In some patients, multiple echogenic layers along the dilated appendix produce the appearance of “onion skin-like” circles and are pathognomonic for mucocele[20,21].
Ascites is a non-specific CT scan finding in pseudo-myxoma peritonei. Visceral scalloping, on the other hand, is a diagnostic sign and distinguishes mucinous from fluid ascites on CT. As the mucin producing cells in pseudomyxoma peritonei are poorly adherent, they are easily dislodged by peristaltic movement and adhere at sites of relative stasis. The pouch of Douglas/rectovesical pouch, right and left subphrenic spaces and surface of the liver and spleen are the commonest sites involved[22].
Right hemicolectomy has been the standard treatment for mucinous and associated appendiceal malignancies[23]. Gonzalez-Moreno et al[14] recently reviewed 501 patients diagnosed with appendiceal epithelial neoplasm from 1983 to 2000. This study showed no survival advantage with right hemicolectomy. The authors suggested the following indications for right hemicolectomy: (1) necessity to clear the primary tumour or achieve complete cytoreduction, (2) lymph node involvement demonstrated by histopathological examination of the appendiceal or ileocolic lymph nodes, (3) a non-mucinous neoplasm identified by histopathological examination.
Recent studies have suggested that an intact mucocoele represents a benign process and does not result in progression of the disease[10]. Rupture and dispersion of mucus or epithelial cells into the peritoneal cavity is more problematic and associated with a poorer prognosis. Extreme care, while handling mucocele during surgery, is imperative to prevent such occurrences. During exci-sion of the mucocele, any mucinous fluid within the abdomen should be carefully examined. If epithelial cells are identified, a diagnosis of pseudomyxoma peritonei syndrome or mucinous peritoneal carcinomatosis of appendiceal origin is established[24]. It is advisable to use a retrievable bag when the laparoscopic approach is used[19,4,25]. Furthermore, it is probably safer to convert to an open procedure if such mucoceles are visualized during a laparo-scopic approach[26].
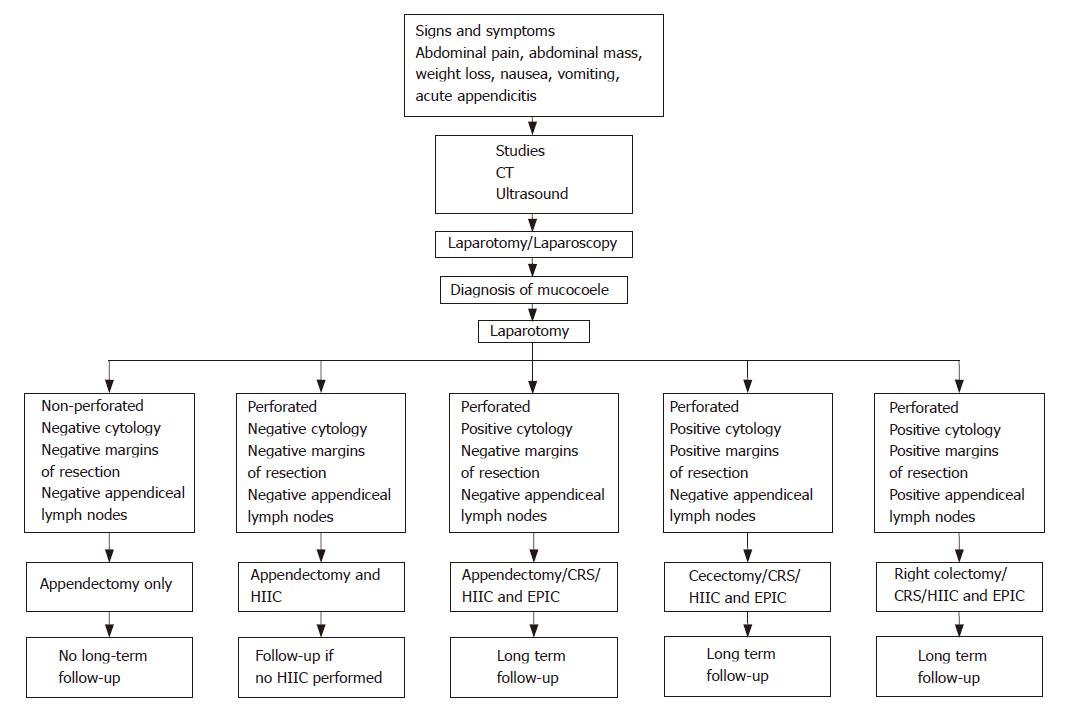
A novel approach to the intraoperative management of patients with peritoneal seeding from a perforated epithe-lial appendiceal malignancy is recommended (Figure 1).
The main goal for treatment of this condition is prevention of locoregional recurrence, rather than systemic disease. Sugarbaker has suggested that a combination of surgery and complete cytoreduction should be followed by intraperitoneal rather than intravenous chemotherapy. Systemic chemotherapy if beneficial is likely to have a transient response[27] and is primarily recommended for patients with extensive peritoneal disease and high grade cystadenocarcinoma[8]. Chemotherapy should be perioperative instead of neoadjuvant. Patient selection should focus on those with negligible residual peritoneal surface disease rather than systemic disease. Peritonectomies are also more favorable than debulking. The recommended chemotherapy regime includes mitomycin followed by five days of post-operative fluorouracil[8]. It is important to refer to a specialist centre early in the course of the disease[5,28,29].
In conclusion, patients with mucocoeles can present with confusing symptoms and indeed may be asymptomatic. Preoperative diagnosis greatly assists in determining appropriate management and minimizing both intra-operative and post-operative complications. Pseudomyxoma is best dealt with in a specialist centre where focus is on prevention of locoregional recurrence. We believe that optimal mana-gement of this condition can greatly prevent serious complications in many cases.
| 1. | Dhage-Ivatury S, Sugarbaker PH. Update on the surgical approach to mucocele of the appendix. J Am Coll Surg. 2006;202:680-684. |
| 2. | Aho AJ, Heinonen R, Laurén P. Benign and malignant mucocele of the appendix. Histological types and prognosis. Acta Chir Scand. 1973;139:392-400. |
| 3. | Landen S, Bertrand C, Maddern GJ, Herman D, Pourbaix A, de Neve A, Schmitz A. Appendiceal mucoceles and pseudomyxoma peritonei. Surg Gynecol Obstet. 1992;175:401-404. |
| 4. | Fann JI, Vierra M, Fisher D, Oberhelman HA, Cobb L. Pseudomyxoma peritonei. Surg Gynecol Obstet. 1993;177:441-447. |
| 5. | Takahashi S, Furukawa T, Ueda J. Case report: Mucocele of the tip of the appendix. Clin Radiol. 1998;53:149-150. |
| 6. | Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol. 2005;12:291-311. |
| 7. | Higa E, Rosai J, Pizzimbono CA, Wise L. Mucosal hyperplasia, mucinous cystadenoma, and mucinous cystadenocarcinoma of the appendix. A re-evaluation of appendiceal "mucocele". Cancer. 1973;32:1525-1541. |
| 8. | Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69-76. |
| 9. | Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, Naessens JM, O'Brien PC, van Heerden JA. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112-119. |
| 10. | Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089-1103. |
| 11. | Haritopoulos KN, Brown DC, Lewis P, Mansour F, Eltayar AR, Labruzzo C, Hakim NS. Appendiceal mucocoele: a case report and review of the literature. Int Surg. 2001;86:259-262. |
| 12. | Pickhardt PJ, Levy AD, Rohrmann CA, Kende AI. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics. 2003;23:645-662. |
| 13. | Stocchi L, Wolff BG, Larson DR, Harrington JR. Surgical treatment of appendiceal mucocele. Arch Surg. 2003;138:585-589; discussion 589-590;. |
| 14. | González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304-311. |
| 15. | Esquivel J, Sugarbaker PH. Clinical presentation of the Pseudomyxoma peritonei syndrome. Br J Surg. 2000;87:1414-1418. |
| 16. | Isaacs KL, Warshauer DM. Mucocele of the appendix: computed tomographic, endoscopic, and pathologic correlation. Am J Gastroenterol. 1992;87:787-789. |
| 17. | Chiou YY, Pitman MB, Hahn PF, Kim YH, Rhea JT, Mueller PR. Rare benign and malignant appendiceal lesions: spectrum of computed tomography findings with pathologic correlation. J Comput Assist Tomogr. 2003;27:297-306. |
| 19. | Lau H, Yuen WK, Loong F, Lee F. Laparoscopic resection of an appendiceal mucocele. Surg Laparosc Endosc Percutan Tech. 2002;12:367-370. |
| 20. | Caspi B, Cassif E, Auslender R, Herman A, Hagay Z, Appelman Z. The onion skin sign: a specific sonographic marker of appendiceal mucocele. J Ultrasound Med. 2004;23:117-121; quiz 122-123. |
| 21. | Kim SH, Lim HK, Lee WJ, Lim JH, Byun JY. Mucocele of the appendix: ultrasonographic and CT findings. Abdom Imaging. 1998;23:292-296. |
| 22. | Sulkin TV, O'Neill H, Amin AI, Moran B. CT in pseudomyxoma peritonei: a review of 17 cases. Clin Radiol. 2002;57:608-613. |
| 23. | HESKETH KT. The management of primary adenocarcinoma of the vermiform appendix. Gut. 1963;4:158-168. |
| 24. | Sugarbaker PH, Ronnett BM, Archer A, Averbach AM, Bland R, Chang D, Dalton RR, Ettinghausen SE, Jacquet P, Jelinek J. Pseudomyxoma peritonei syndrome. Adv Surg. 1996;30:233-280. |
| 25. | Miraliakbari R, Chapman WH. Laparoscopic treatment of an appendiceal mucocele. J Laparoendosc Adv Surg Tech A. 1999;9:159-163. |
| 26. | González Moreno S, Shmookler BM, Sugarbaker PH. Appendiceal mucocele. Contraindication to laparoscopic appendectomy. Surg Endosc. 1998;12:1177-1179. |
| 27. | Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300-308. |
| 28. | Loungnarath R, Causeret S, Bossard N, Faheez M, Sayag-Beaujard AC, Brigand C, Gilly F, Glehen O. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum. 2005;48:1372-1379. |
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y