Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.780
Revised: July 9, 2005
Accepted: July 12, 2005
Published online: February 7, 2006
AIM: To investigate the ultra-structural changes and heat shock protein 70 (HSP70) expression in the pancreas of rats with endotoxic shock and to detect their possible relationship.
METHODS: A total of 33 Wistar rats were randomly divided into three groups: control group (given normal saline), small dose lipopolysaccharide (LPS) group (given LPS 5 mg/kg) and large dose LPS group (given LPS 10 mg/kg). Pancreas was explanted to detect the ultra-structural changes by TEM and the HSP70 expression by immunohistochemistry and Western blot.
RESULTS: Rats given small doses of LPS showed swelling and loss of mitochondrial cristae of acinar cells and increased number of autophagic vacuoles in the cytoplasm of acinar cells. Rats given large doses of LPS showed swelling, vacuolization, and obvious myeloid changes of mitochondrial cristae of acinar cells, increased number of autophagic vacuoles in the cytoplasm of acinar cells. HSP70 expression was increased compared to the control group (P<0.05).
CONCLUSION: Small doses of LPS may induce stronger expression of HSP70, promote autophagocytosis and ameliorate ultra-structural injuries.
- Citation: Wang XL, Li Y, Kuang JS, Zhao Y, Liu P. Increased heat shock protein 70 expression in the pancreas of rats with endotoxic shock. World J Gastroenterol 2006; 12(5): 780-783
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/780.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.780
Septic shock is a clinical syndrome resulting from the systemic response of the body to infection. Septic shock is characterized by hypoperfusion of major organs, leading to multiple organ failure and finally to death. Though advances have been achieved in supportive and anti-microbial treatment, septic shock continues to be a common cause of intensive care with the mortality rate being approximately 40%[1]. Sepsis is associated with secretary pancreatic dysfunction[2].
Heat shock proteins (HSPs) are highly conserved molecules present in both prokaryotic and eukaryotic cells and play an important role in cellular function and stress conditions. HSPs function as molecular chaperones and are categorized into HSP90, HSP70, HSP60, HSP20, and HSP8.5 on the basis of their molecular weight. HSP70 proteins constitute the central part of the chaperone system. In mammals, two isoforms of HSP70 exist in cytoplasm: a 73-ku constitutively expressed form (HSC70) and a 72-ku stress-inducible form (HSP70). HSPs are usually constitutively present at low concentrations in cells, but are actively synthesized to reach much higher concentrations as cells react to aggressive situations[3].
This study was to investigate the effect of different doses of LPS on the expression of HSP70 in a model of LPS-induced septic shock.
A total of 33 Wistar rats weighing 250±10 g were supplied by China Medical University Animal Department. All animals received humane care.
A total of 33 Wistar rats were randomly divided into three groups. Small dose LPS group (n = 12) was given a single dose of 5 mg/kg LPS, large dose LPS group (n =12) was given 10 mg/kg LPS, control group (n = 9) was given the same volume of 0.9% normal saline. All rats were anesthetized with sodium pentobarbital (36 mg/kg). LPS (SIGMA, O127B8) was given via the femoral vein. A catheter was connected to the femoral artery to measure the mean arterial pressure. Death occurred after 3.5 h. The rats that survived were killed at 6 h. Pancreas were removed immediately and stored at -70 °C for later use.
Pancreatic samples were fixed in neutral buffered formalin, embedded in paraffin, sectioned, and mounted on slides. After being deparaffinized, endogenous peroxidase activity was quenched with 10% H2O2 for 10 min. After being blocked with goat serum, the sections were incubated with anti-mouse IgG polyclonal HSP70 antibody at 37 °C for 90 min, followed by incubation with S-A/HRP at 37 °C for 20 min. The Ag-Ab complex was visualized using diaminobenzidine method. Slides were then counterstained with hematoxylin for 1 min. After dehydration, the slides were coverslipped. To ensure specificity of the immunoreactions, control sections were subjected to the same immunohistochemical method with the exception that the primary Ab antibody was replaced with PBS.
Pancreatic tissue was cut into 1 mm × 1 mm × 1 mm pieces and placed in a freshly prepared fixative buffer (2.5% gluteraldehyde) at 4 °C for 2 h. After being fixed in 1% osmium tetroxide at 4 °C for 1 h, the tissue was exposed twice to an increased concentration of ethanol (30%, 50%, 70%, and 99.5%) at 5-min intervals for partial dehydration. Tissue was embedded with resin in gelatin capsules and incubated at 55-60 °C for 24 h. Ultra-thin (70 nm) sections were cut and transferred to 300-mesh nickel griads. After being stained with tannic acid, saturated uranyl acetate in 50% alcohol and 0.01% lead citrate, the samples were examined under transmission electron microscope (JEM-1200EX, Tokyo, Japan).
Tissues were sonicated on ice for approximately 10 min until the tissues were completely homogenized in a solution containing 2 mmol/L EDTA, 2 mmol/L EGTA, 0.2 mmol/L NaCl, 20 mmol/L Tris-HCl, 1% Triton, 1 mmol/L DTT, and 1 mmol/L aprotinin. The homogenate was then centrifuged at 12000 g for 60 min at 4 °C and the resulting supernatant fraction was transferred to a fresh tube.
An equal amount (30 µg) of protein extracts was loaded and separated by 8% SDS-polyacrylamide gel electrophoresis (PAGE). After electrophoresis, the proteins on the gel were transferred to polyvinylidene difluoride (PVDF) membrane. After being blocked with 5% nonfat dry milk at 4 °C overnight, the PVDF membranes were incubated with anti-rabbit polyclonal antibody of HSP70 diluted 1:200 with TPBS at 37 °C for 2 h. Then the blots were reacted with goat anti-rabbit antibody at room temperature for 2 h. Pre-stained molecular mass was used to estimate the positions of various proteins on the gel. Blots were stripped and incubated with a monoclonal Ab against β-actin (Sigma) to confirm that an equal protein was loaded. The target was then detected by enhanced chemiluminescence and exposed to X-ray film for appropriate time. The pictures were analyzed by GIS TANON.
All data were expressed as mean ± SD. P < 0.05 was considered statistically significant.
No obvious ultra-structural damage was found in the control group. Swelling and loss of mitochondrial cristae of acinar cells and increased number of autophagic vacuoles in the cytoplasm of acinar cells were found in small dose LPS group. Swelling, vacuolization, and obvious myeloid changes of mitochondrial cristae of acinar cells and increased number of autophagic vacuoles in the cytoplasm of acinar cells were found in large dose LPS group (figure 2).
HSP70 was expressed at a low level in pancreatic acinar cells of the control group. After injection of LPS, HSP70 was highly expressed in the cytoplasm and nuclei of acinar cells and endothelia of blood vessels. Immunohistochemical examination showed overexpression of HSP70 in the vascular endothelial cells and cytoplasm of pancreatic tissue of the small dose LPS group. There were no significant differences between the two groups (Table 1).
| Groups | n | – | + | ++ | +++ |
| LPS5 mg/kg | 12 | 2 | 0 | 3 | 7 |
| LPS10 mg/kg | 12 | 4 | 1 | 3 | 4 |
| Control group | 9 | 2 | 6 | 1 | 0 |
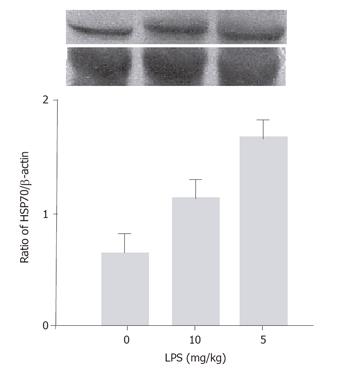
HSP70 expression in the control group was 112 ± 18.9 (0.62 ± 0.04). HSP70 expression in the large dose LPS group was 169 ± 13.4 (0.974 ± 0.04). HSP70 expression in the small dose LPS group was 211 ± 11.5 (1.34 ± 0.17) (Figure 1 and 3). β-Actin expression was constant, indicating the equal protein loading on the blots. HSP70 expression increased compared to the control group (P < 0.05).
This study showed that LPS could induce HSP70 expression in the pancreas of rats with endotoxic shock and the upregulation of HSP70 was associated with the doses of LPS and the extent of pancreatic ultra-structural injury. Endotoxin caused mitochondrial changes in the acinar cells of the pancreas and large doses of endotoxin led to myeloid changes of mitochondria. Small doses of LPS induced obvious overexpression of HSP70 and autophagocytosis, suggesting that high level of HSP70 promotes degradation of damaged proteins and ameliorates ultra-structural damages.
HSPs can protect cells from injury induced by environmental challenges, such as hypoxia, ischemia, high temperature, endotoxin, infection, and fever[4]. The protective effects of HSPs have been confirmed in various animal models of sepsis[5]. Several studies have demonstrated that a preceding HSP reaction reduces both mortality and organ dysfunction in experimentally induced severe sepsis[6]. Induction of HSPs in response to stress correlates with increased resistance to subsequent cellular damage. HSP70 is believed to act in a chaperone-like manner aiding in the passage of proteins across membrane barriers, preventing misfolding of newly synthesized proteins, facilitating elimination of improperly assembled, misfolded or aggregated proteins[7]. HSP70 contributes to the delivery of dysfunctional proteins to lysosomes for proteolytic degradation[8]. HSP70 is also known to facilitate antigen presentation in cells such as macrophages and dendrites[9]. Elevations in intracellular HSP levels have been shown to improve cell tolerance to inflammatory cytokines, such as TNF-α and IL-1β[10]. TNF-α and IL-1β are thought to be the main mediators in the pathogenesis of septic shock[11]. HSP70 expression protects the lung against ventilator-induced lung injury by decreasing cytokine transcription[12].
Mitochondrial oxidative phosphorylation is responsible for over 90% of total body oxygen consumption and ATP generation. HSP70 upregulation protects mitochondrial function after ischemia-reperfusion injury[13]. Increased levels of HSP70 through gene transfection lead to a greater protection of mitochondrial function after ischemia-reperfusion in a donor heart preservation protocol[13]. This may be one mechanism by which HSP70 overexpression leads to a better protection. Overexpression of HSP70 in the whole pancreas can protect mitochondrial integrity[14]. It was reported that structural deformity and decrease of respiratory chain enzyme activity in mitochondria and decline of ATP content are highly correlated with the deterioration of cardiac function during sepsis[15]. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis[16], thus achieving a protective goal.
HSP70 protection against LPS is most probably mediated through the modulation of iNOS activation and the subsequent decreased synthesis of nitric oxide (NO)[17]. NO plays an important role in the pathogenesis of septic shock[18]. A key role of HSP70 in the natural resistance of human beta cells against NO-induced injury is by preserving mitochondrial function[19]. Constitutive expression of HSP70 in human beta cells is essential for the natural resistance against NO-induced injury[19]. HSP70 prevents secretogogue-induced cell injury in the pancreas[20]. Hyperthermia protects against arginine-induced pancreatitis and induces HSP70 in the pancreas[21].
After entering blood, endotoxin combines with CD14[22]. Both CD14 and TLR2 are receptors of LPS[22]. TLR-4 and CD14 are involved in HSP70-mediated proinflammatory responses[23] and in HSP70-mediated activation of innate immunity[24]. Further investigation is necessary to elucidate the mechanism underlying the induction of endotoxin by HSP70.
| 1. | Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 506] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 2. | Tribl B, Sibbald WJ, Vogelsang H, Spitzauer S, Gangl A, Madl C. Exocrine pancreatic dysfunction in sepsis. Eur J Clin Invest. 2003;33:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing 'heat shock' proteins. J Cell Sci. 2002;115:2809-2816. [PubMed] |
| 4. | Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3762] [Cited by in RCA: 3686] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 5. | Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Bruemmer-Smith S, Stüber F, Schroeder S. Protective functions of intracellular heat-shock protein (HSP) 70-expression in patients with severe sepsis. Intensive Care Med. 2001;27:1835-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Frossard JL, Bhagat L, Lee HS, Hietaranta AJ, Singh VP, Song AM, Steer ML, Saluja AK. Both thermal and non-thermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 8. | Agarraberes FA, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491-2499. [PubMed] |
| 9. | Kuppner MC, Gastpar R, Gelwer S, Nössner E, Ochmann O, Scharner A, Issels RD. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602-1609. [PubMed] |
| 10. | Jäättelä M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993;177:231-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Schirmer WJ, Schirmer JM, Fry DE. Recombinant human tumor necrosis factor produces hemodynamic changes characteristic of sepsis and endotoxemia. Arch Surg. 1989;124:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Vreugdenhil HA, Haitsma JJ, Jansen KJ, Zijlstra J, Plötz FB, Van Dijk JE, Lachmann B, Van Vught H, Heijnen CJ. Ventilator-induced heat shock protein 70 and cytokine mRNA expression in a model of lipopolysaccharide-induced lung inflammation. Intensive Care Med. 2003;29:915-922. [PubMed] |
| 13. | Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 2001;104:I303-I307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Sammut IA, Jayakumar J, Latif N, Rothery S, Severs NJ, Smolenski RT, Bates TE, Yacoub MH. Heat stress contributes to the enhancement of cardiac mitochondrial complex activity. Am J Pathol. 2001;158:1821-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1087] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 16. | Chen HW, Hsu C, Lu TS, Wang SJ, Yang RC. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock. 2003;20:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lau SS, Griffin TM, Mestril R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1439-H1445. [PubMed] |
| 18. | Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis--an overview. Acta Anaesthesiol Scand. 1999;43:275-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Burkart V, Liu H, Bellmann K, Wissing D, Jäättela M, Cavallo MG, Pozzilli P, Briviba K, Kolb H. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000;275:19521-19528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest. 2000;106:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Tashiro M, Ernst SA, Edwards J, Williams JA. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Wong PM, Chugn SW, Sultzer BM. Genes, receptors, signals and responses to lipopolysaccharide endotoxin. Scand J Immunol. 2000;51:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028-15034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1144] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 24. | Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 281] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Cao L