Published online Aug 7, 2006. doi: 10.3748/wjg.v12.i29.4609
Revised: March 23, 2006
Accepted: March 27, 2006
Published online: August 7, 2006
Slow transit constipation has been traditionally considered and classified as a functional disorder. However, clinical and manometric evidence has been accumulating that suggests how most of the motility alterations in STC might be considered of neuropathic type.In addition, further investigations showed that subtle alterations of the enteric nervous system, not evident to conventional histological examination, may be present in these patients. In the present article we will discuss these evidences, and will try to put them in relation with the abnormal motor function of the large bowel documented in this pathological condition.
- Citation: Bassotti G, Villanacci V. Slow transit constipation: A functional disorder becomes an enteric neuropathy. World J Gastroenterol 2006; 12(29): 4609-4613
- URL: https://www.wjgnet.com/1007-9327/full/v12/i29/4609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i29.4609
In gastroenterology practice, the most frequently encountered disorders are represented by those related to an abnormal function of the abdominal viscera, the so-called functional diseases[1,2]. The term “functional” defines several variable combinations of chronic or recurrent gastrointestinal symptoms that do not have an identified underlying pathophysiology[3], and that are often also labelled as “idiopathic”. Thus, the definition of functional relies on the exclusion of an organic disease, and it is consequently thought to be related to abnormalities of its physiological properties or functions.
Among the so-called functional disorders, one of the most common is functional constipation, whose diagnostic criteria according to Rome II classification[4] are reported in Table 1. Patients with functional constipation may be further classified in three subgroups: normal transit constipation, disorders of defecatory or rectal evacuation (outlet obstruction), and slow transit constipation (STC)[5,6]. This latter condition affects mainly women, it is characterized by an often intractable constipation , a heavily delayed colonic transit up to true colonic inertia[7,8], and it is usually attributed to disorders of colonic motor function[9,10]. Indeed, several abnormal motor aspects have been described in STC, such as alterations of rectosigmoid contractile activity[11], decreased colonic propulsive function[12,13], abnormal response to food ingestion[14,15], and overall reduced electrical or motor activity of the large bowel[16,17]. It must also be taken into account that a further cause of delayed colonic transit may be due to the association with pelvic floor dyssynergia; this condition must be identified, since it is amenable of biofeedback treatment, which can normalize colonic transit[18].
| Two or more of the following for at least 12 wk (not necessarily consecutive) in the preceding 12 mo: |
| • Straining during > 25% of bowel movements; |
| • Lumpy/hard stools for > 25% of bowel movements; |
| • Sensation of incomplete evacuation for > 25% of bowel movements; |
| • Sensation of anorectal blockage for > 25% of bowel movements; |
| • Manual manoeuvres to facilitate > 25% of bowel movements (e.g., digital evacuation or support of the pelvic floor); |
| • < 3 bowel movements per week Loose stools are not present, and there are insufficient criteria for irritable bowel syndrome |
However, we still do not know how and why these abnormalities are present in STC patients. In recent years, clinical and manometric evidence has been accumulated that suggests how most of the motility alterations in STC might be considered as a neuropathic type. Moreover, other data showed that some subtle alterations of the enteric nervous system, not evident to conventional histological examination, may be present in these patients. In the present article we will discuss these evidences, and will try to put them in relation with the abnormal motor function of the large bowel documented in this pathological condition.
In several patients with STC subclinical features of autonomic neuropathy may be present[19], and other studies described selective sensory and autonomic neuropathy in these subjects[20], often with a positive family history, suggesting a genetic basis for this condition[21]. Animal studies might help in elucidating these issues: for instance, transgenic mice with a targeted deletion of neurturin (a neurotrophin) display clinical and tissue phenotype similar to that found in STC, and display associated parasympathetic deficits[22].
Moreover, since in a sizable proportion of STC patients the symptoms start after pelvic surgical procedures[23-25] or following childbirth[26] it has been hypothesized (although the anatomic proof has never been given) that pelvic nerve injury may occur following hysterectomy and childbirth, and that STC could be considered a disorder of pelvic autonomic nerves at least in a subgroup of patients[27].
Several manometric studies carried out in patients with STC have reported findings that suggest the presence of neuropathic-type abnormalities. Such abnormalities have been described in many instances with respect to the periodic motor activity of the rectosigmoid area (including the so-called rectal motor complex), that appears either decreased or disorganized[28-30], the contractile colonic motor response following intravenous cholinergic stimulation, that in results impaired[31], the early motor response following ingestion of a meal, reported as decreased or absent[32], the overall daily colonic motility, usually decreased to a lesser or greater extent[33,34], the daily organization of regular contractile colonic patterns, that is often impaired[35], and the lack of propulsive response to intraluminal instillation of bisacodyl (a powerful stimulant of mass movements in healthy subjects)[36].
It is worth noting that neuropathic-type abnormalities in patients with STC are not necessarily confined to the large bowel, but may be also documented in other viscera such as the esophagus[37,38], the stomach[39,40], the gallbladder[41], and especially the small bowel[42-45], suggesting the presence of a pan-enteric motor disorder in these patients, particularly in those with more severe symptoms.
Most of the pathologic descriptions related to STC are pertinent to the large bowel, and only sporadic reports from other gastrointestinal viscera (terminal ileum) exist. Therefore, the discussion of pathological findings will focus on the colonic studies.
Most studies employing routine light microscopy have failed to identify consistent abnormalities of the enteric nervous system (ENS) in patients with STC[46-50], apart from the presence of melanosis coli. However, we have recently demonstrated that melanosis coli per se does not have any relationship with colonic ENS abnormalities (in particular with the loss of enteric neurons, as hypothesized in older studies only evaluating the submucosa) in these patients[51].
Morphological abnormalities of colonic innervation have been described in STC patients using the silver staining technique introduced by Smith[52]. These studies have generally reported a reduction in the total number of argyrophilic neurones and morphological neuronal and/or axonal abnormalities[53,54]. However, the silver staining technique has been subsequently replaced by more modern and reliable immunohistochemical methods (see below).
Concerning the hypothesized imbalance of enteric neurotransmitters or the enzyme markers (mostly neuro-peptides) in STC, the various studies (using immuno-staining, immunoassays, or both methods) have frequently yielded inconsistent results. In fact, looking at the findings related to the most commonly investigated neuropeptides (VIP, substance P, neuropeptide Y and 5-HT), decreased, increased or unchanged levels or immunoreactivity has been described in these patients[55-59]. Overall, on the basis of the above reports it might be stated that it is unlikely that alterations of the enteric neurotransmitterns may play a major role in the pathophysiology of STC. However, more recent observations suggest that an excessive production of nitric oxide in the colonic myenteric plexus of patients with STC could play a pathophysiological role, concurring in the persistent inhibition of contractions[60,61].
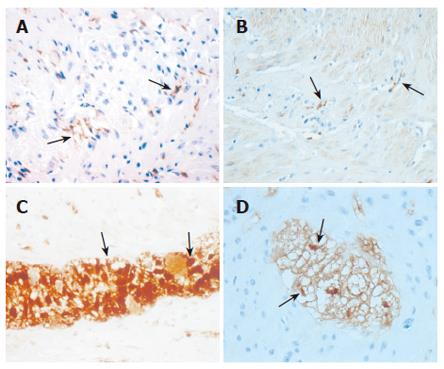
More consistent results have been reported with respect to enteric neurons, interstitial cells of Cajal (ICC), and enteric glial cells (EGC). In fact, a decrease of enteric neural elements (neurons and/or neurofilaments) seems to be a constant feature in studies evaluating patients with STC severe enough to require surgery for symptoms’ relief[62-66], and these abnormalities are often associated with a reduced number of ICC[67-71], although this latter finding is not constantly present[72]. We have recently shown in a relatively large and homogeneous group of patients with severe and intractable STC, compared to age-matched controls, that the ICC are significantly decreased in patients (Figures 1A and B), that the enteric neuronal loss may be partially due to apoptotic phenomena, and that these patients display a significantly decreased number of EGC with respect to controls (Figures 1C and D), in both the submucosal and myenteric plexuses[73].
On the basis of the above evidences, it seems now possible to track a link between the clinical picture, represented by severe constipation with heavily delayed colonic transit, often refractory to medical treatment, the instrumental manometric findings, that mostly show impaired motility and propulsive activity of the large bowel (sometimes with the participation of the upper gastrointestinal segments too), and the abnormalities of the colonic ENS.
The neuronal loss is likely to affect the motor activity of the large bowel, reducing the likelihood of enteric neurotransmission. This defect is then strengthened by the concomitant reduction of the number of ICC, a cell population of paramount importance for the correct homeostasis of gastrointestinal motility. In fact, the primary role of ICC as intestinal pacemakers has been established in experimental animal models, where it has been shown that a lack of ICC networks leads to the absence of slow waves and is accompanied by delayed or absent intestinal motility[74,75]. A decreased ICC function might therefore impair the colonic electrical slow wave activity, thereby affecting the contractile response and causing delayed transit in STC patients. In addition, it has been recently demonstrated that in patients with STC the expression of c-kit mRNA and c-kit protein is significantly decreased compared to controls, suggesting that alterations in the c-kit signal pathway may play an important role in ICC reduction in such patients[76].
An interesting findings, never described before, was the significant decrease of EGC in both the myenteric and submucous plexuses in STC patients compared to controls. This cell population originates from the neural crest and provides both mechanical and physiological support for neuronal elements[77]. The chief known function of the glia in the adult is the formation of myelin sheaths around axons, allowing the fast connections essential for the nervous system function. Moreover, EGC maintain the appropriate concentrations of ions and neurotransmitters in the neuronal environment and are essential regulators of the formation, maintenance and function of synapses, the key functional units of the nervous system[78,79]. Since EGC are thought to act as intermediaries in enteric neurotransmission[80], it is likely that their decrease could synergistically act in further weakening the already precarious neuroenteric balance due to the decrease of neuronal elements and ICC found in patients with STC.
The case for reclassifying STC other than an “idiopathic” or “functional” disorder is built. In fact, as seen above, clinical, instrumental, and pathological evidences exist that all point toward to a (perhaps) more precise definition of this condition as a true enteric neuropathy. It is probably too early to target STC with a different label, but at least we are now aware of some basic pathophysiological mechanisms potentially responsible for the symptoms and the manometric abnormalities found in this condition. Moreover, apart from mere semantic considerations, the demonstration of such background abnormalities might reveal useful for more targeted therapeutic approaches. For instance, in a mouse model the blockage of Kit receptors caused transdifferentiation of intestinal ICC to a smooth muscle phenotype[81]: if the same would occur in the human colon and if ICC do not die in STC but rather redifferentiate, it might be possible to create conditions that would shift the phenotype back toward ICC.
In conclusion, the advancement of our knowledge of the possible pathophysological mechanisms of “functional” disorders is important for a more correct clinical and therapeutic approach. Further studies are obviously needed before we can drop the “idiopathic” label from these disorders.
S- Editor Wang J L- Editor Chiarioni G E- Editor Ma WH
| 1. | Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20 Suppl 7:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 248] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Williams M, Budavari A, Olden KW, Jones MP. Psychosocial assessment of functional gastrointestinal disorders in clinical practice. J Clin Gastroenterol. 2005;39:847-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 3. | Corazziari E. Definition and epidemiology of functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:613-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 4. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 832] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 5. | Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 518] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 6. | Prather CM. Subtypes of constipation: sorting out the confusion. Rev Gastroenterol Disord. 2004;4 Suppl 2:S11-S16. [PubMed] |
| 7. | Knowles CH, Martin JE. Slow transit constipation: a model of human gut dysmotility. Review of possible aetiologies. Neurogastroenterol Motil. 2000;12:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Bassotti G, Roberto GD, Sediari L, Morelli A. Toward a definition of colonic inertia. World J Gastroenterol. 2004;10:2465-2467. [PubMed] |
| 9. | Bharucha AE, Phillips SF. Slow transit constipation. Gastroenterol Clin North Am. 2001;30:77-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 10. | Bassotti G, de Roberto G, Castellani D, Sediari L, Morelli A. Normal aspects of colorectal motility and abnormalities in slow transit constipation. World J Gastroenterol. 2005;11:2691-2696. [PubMed] |
| 11. | Preston DM, Lennard-Jones JE. Pelvic motility and response to intraluminal bisacodyl in slow-transit constipation. Dig Dis Sci. 1985;30:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 12. | Kamm MA, van der Sijp JR, Lennard-Jones JE. Observations on the characteristics of stimulated defaecation in severe idiopathic constipation. Int J Colorectal Dis. 1992;7:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 13. | Bassotti G, Chistolini F, Nzepa FS, Morelli A. Colonic propulsive impairment in intractable slow-transit constipation. Arch Surg. 2003;138:1302-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 14. | Bassotti G, Imbimbo BP, Betti C, Dozzini G, Morelli A. Impaired colonic motor response to eating in patients with slow-transit constipation. Am J Gastroenterol. 1992;87:504-508. [PubMed] |
| 15. | Björnsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL. Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT(3) pathways. Am J Physiol Gastrointest Liver Physiol. 2002;283:G400-G407. [PubMed] |
| 16. | Schang JC, Devroede G, Duguay C, Hémond M, Hébert M. [Constipation caused by colonic inertia and distal obstruction: electromyographic study]. Gastroenterol Clin Biol. 1985;9:480-485. [PubMed] |
| 17. | Bassotti G, Gaburri M, Imbimbo BP, Rossi L, Farroni F, Pelli MA, Morelli A. Colonic mass movements in idiopathic chronic constipation. Gut. 1988;29:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 175] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 18. | Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 246] [Article Influence: 11.7] [Reference Citation Analysis (2)] |
| 19. | Altomare D, Pilot MA, Scott M, Williams N, Rubino M, Ilincic L, Waldron D. Detection of subclinical autonomic neuropathy in constipated patients using a sweat test. Gut. 1992;33:1539-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 20. | Raethjen J, Pilot MA, Knowles C, Warner G, Anand P, Williams N. Selective autonomic and sensory deficits in slow transit constipation. J Auton Nerv Syst. 1997;66:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 21. | Knowles CH, Scott SM, Wellmer A, Misra VP, Pilot MA, Williams NS, Anand P. Sensory and autonomic neuropathy in patients with idiopathic slow-transit constipation. Br J Surg. 1999;86:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 22. | Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM Jr. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 253] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 23. | Roe AM, Bartolo DC, Mortensen NJ. Slow transit constipation. Comparison between patients with or without previous hysterectomy. Dig Dis Sci. 1988;33:1159-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 24. | Vierhout ME, Schreuder HW, Veen HF. Severe slow-transit constipation following radical hysterectomy. Gynecol Oncol. 1993;51:401-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 25. | MacDonald A, Baxter JN, Finlay IG. Idiopathic slow-transit constipation. Br J Surg. 1993;80:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 26. | MacDonald A, Baxter JN, Bessent RG, Gray HW, Finlay IG. Gastric emptying in patients with constipation following childbirth and due to idiopathic slow transit. Br J Surg. 1997;84:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 27. | Knowles CH, Scott SM, Lunniss PJ. Slow transit constipation: a disorder of pelvic autonomic nerves. Dig Dis Sci. 2001;46:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 28. | Waldron DJ, Kumar D, Hallan RI, Wingate DL, Williams NS. Evidence for motor neuropathy and reduced filling of the rectum in chronic intractable constipation. Gut. 1990;31:1284-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 29. | Bassotti G, Betti C, Pelli MA, Morelli A. Prolonged (24-hour) manometric recording of rectal contractile activity in patients with slow transient constipation. Digestion. 1991;49:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 30. | Rao SS, Sadeghi P, Batterson K, Beaty J. Altered periodic rectal motor activity: a mechanism for slow transit constipation. Neurogastroenterol Motil. 2001;13:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 31. | Bassotti G, Chiarioni G, Imbimbo BP, Betti C, Bonfante F, Vantini I, Morelli A, Whitehead WE. Impaired colonic motor response to cholinergic stimulation in patients with severe chronic idiopathic (slow transit type) constipation. Dig Dis Sci. 1993;38:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 32. | Bassotti G, Morelli A, Whitehead WE. Abnormal rectosigmoid myoelectric response to eating in patients with severe idiopathic constipation (slow-transit type). Dis Colon Rectum. 1992;35:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 33. | Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Shafik A, Shafik AA, El-Sibai O, Mostafa RM. Electric activity of the colon in subjects with constipation due to total colonic inertia: an electrophysiologic study. Arch Surg. 2003;138:1007-1011; discussion 1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Bassotti G, Chistolini F, Battaglia E, Chiarioni G, Nzepa FS, Dughera L, deRoberto G, Emanuelli G, Morelli A. Are colonic regular contractile frequency patterns in slow transit constipation a relevant pathophysiological phenomenon. Dig Liver Dis. 2003;35:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 36. | Bassotti G, Chiarioni G, Germani U, Battaglia E, Vantini I, Morelli A. Endoluminal instillation of bisacodyl in patients with severe (slow transit type) constipation is useful to test residual colonic propulsive activity. Digestion. 1999;60:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 37. | Watier A, Devroede G, Duranceau A, Abdel-Rahman M, Duguay C, Forand MD, Tétreault L, Arhan P, Lamarche J, Elhilali M. Constipation with colonic inertia. A manifestation of systemic disease. Dig Dis Sci. 1983;28:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 38. | Altomare DF, Portincasa P, Rinaldi M, Di Ciaula A, Martinelli E, Amoruso A, Palasciano G, Memeo V. Slow-transit constipation: solitary symptom of a systemic gastrointestinal disease. Dis Colon Rectum. 1999;42:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (3)] |
| 39. | van der Sijp JR, Kamm MA, Nightingale JM, Britton KE, Granowska M, Mather SJ, Akkermans LM, Lennard-Jones JE. Disturbed gastric and small bowel transit in severe idiopathic constipation. Dig Dis Sci. 1993;38:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 40. | Mollen RM, Hopman WP, Oyen WJ, Kuijpers HH, Edelbroek MA, Jansen JB. Effect of subtotal colectomy on gastric emptying of a solid meal in slow-transit constipation. Dis Colon Rectum. 2001;44:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 41. | Gunay A, Gurbuz AK, Narin Y, Ozel AM, Yazgan Y. Gallbladder and gastric motility in patients with idiopathic slow-transit constipation. South Med J. 2004;97:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 42. | Bassotti G, Stanghellini V, Chiarioni G, Germani U, De Giorgio R, Vantini I, Morelli A, Corinaldesi R. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci. 1996;41:1999-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 43. | Glia A, Lindberg G. Antroduodenal manometry findings in patients with slow-transit constipation. Scand J Gastroenterol. 1998;33:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 44. | Scott SM, Picon L, Knowles CH, Fourquet F, Yazaki E, Williams NS, Lunniss PJ, Wingate DL. Automated quantitative analysis of nocturnal jejunal motor activity identifies abnormalities in individuals and subgroups of patients with slow transit constipation. Am J Gastroenterol. 2003;98:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Panagamuwa B, Kumar D, Ortiz J, Keighley MR. Motor abnormalities in the terminal ileum of patients with chronic idiopathic constipation. Br J Surg. 1994;81:1685-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 46. | Preston DM, Hawley PR, Lennard-Jones JE, Todd IP. Results of colectomy for severe idiopathic constipation in women (Arbuthnot Lane's disease). Br J Surg. 1984;71:547-552. [PubMed] |
| 47. | Kamm MA, Hawley PR, Lennard-Jones JE. Outcome of colectomy for severe idiopathic constipation. Gut. 1988;29:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 48. | Walsh PV, Peebles-Brown DA, Watkinson G. Colectomy for slow transit constipation. Ann R Coll Surg Engl. 1987;69:71-75. [PubMed] |
| 49. | Bassotti G, Betti C, Pelli MA, Morelli A. Extensive investigation on colonic motility with pharmacological testing is useful for selecting surgical options in patients with inertia colica. Am J Gastroenterol. 1992;87:143-147. [PubMed] |
| 50. | Redmond JM, Smith GW, Barofsky I, Ratych RE, Goldsborough DC, Schuster MM. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995;90:748-753. [PubMed] |
| 51. | Villanacci V, Bassotti G, Cathomas G, Maurer CA, Di Fabio F, Fisogni S, Cadei M, Mazzocchi A, Salerni B. Is pseudomelanosis coli a marker of colonic neuropathy in severely constipated patients. Histopathology. 2006;49:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 52. | Smith B. Myenteric plexus in Hirschsprung's disease. Gut. 1967;8:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 53. | Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE 2nd. Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology. 1985;88:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Zenilman ME, Dunnegan DL, Soper NJ, Becker JM. Successful surgical treatment of idiopathic colonic dysmotility. The role of preoperative evaluation of coloanal motor function. Arch Surg. 1989;124:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 55. | Koch TR, Carney JA, Go L, Go VL. Idiopathic chronic constipation is associated with decreased colonic vasoactive intestinal peptide. Gastroenterology. 1988;94:300-310. [PubMed] |
| 56. | Dolk A, Brodén G, Holmström B, Johansson C, Schultzberg M. Slow transit chronic constipation (Arbuthnot Lane's disease). An immunohistochemical study of neuropeptide-containing nerves in resected specimens from the large bowel. Int J Colorectal Dis. 1990;5:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 57. | Lincoln J, Crowe R, Kamm MA, Burnstock G, Lennard-Jones JE. Serotonin and 5-hydroxyindoleacetic acid are increased in the sigmoid colon in severe idiopathic constipation. Gastroenterology. 1990;98:1219-1225. [PubMed] |
| 58. | Tzavella K, Riepl RL, Klauser AG, Voderholzer WA, Schindlbeck NE, Müller-Lissner SA. Decreased substance P levels in rectal biopsies from patients with slow transit constipation. Eur J Gastroenterol Hepatol. 1996;8:1207-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 59. | Sjölund K, Fasth S, Ekman R, Hultén L, Jiborn H, Nordgren S, Sundler F. Neuropeptides in idiopathic chronic constipation (slow transit constipation). Neurogastroenterol Motil. 1997;9:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Cortesini C, Cianchi F, Infantino A, Lise M. Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig Dis Sci. 1995;40:2450-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 61. | Tomita R, Fujisaki S, Ikeda T, Fukuzawa M. Role of nitric oxide in the colon of patients with slow-transit constipation. Dis Colon Rectum. 2002;45:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 62. | Schouten WR, ten Kate FJ, de Graaf EJ, Gilberts EC, Simons JL, Klück P. Visceral neuropathy in slow transit constipation: an immunohistochemical investigation with monoclonal antibodies against neurofilament. Dis Colon Rectum. 1993;36:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 63. | Park HJ, Kamm MA, Abbasi AM, Talbot IC. Immunohistochemical study of the colonic muscle and innervation in idiopathic chronic constipation. Dis Colon Rectum. 1995;38:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 64. | Porter AJ, Wattchow DA, Hunter A, Costa M. Abnormalities of nerve fibers in the circular muscle of patients with slow transit constipation. Int J Colorectal Dis. 1998;13:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 65. | Faussone-Pellegrini MS, Infantino A, Matini P, Masin A, Mayer B, Lise M. Neuronal anomalies and normal muscle morphology at the hypomotile ileocecocolonic region of patients affected by idiopathic chronic constipation. Histol Histopathol. 1999;14:1119-1134. [PubMed] |
| 66. | Wedel T, Roblick UJ, Ott V, Eggers R, Schiedeck TH, Krammer HJ, Bruch HP. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 67. | He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 289] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 68. | Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 69. | Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 70. | Tong WD, Liu BH, Zhang LY, Zhang SB, Lei Y. Decreased interstitial cells of Cajal in the sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2004;19:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 71. | Lee JI, Park H, Kamm MA, Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 2005;20:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 72. | Toman J, Turina M, Ray M, Petras RE, Stromberg AJ, Galandiuk S. Slow transit colon constipation is not related to the number of interstitial cells of Cajal. Int J Colorectal Dis. 2006;21:527-532. [PubMed] |
| 73. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 74. | Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91-97. [PubMed] |
| 75. | Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1081] [Article Influence: 34.9] [Reference Citation Analysis (3)] |
| 76. | Tong WD, Liu BH, Zhang LY, Xiong RP, Liu P, Zhang SB. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2005;20:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 77. | Rühl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 78. | Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 79. | Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004;36:1861-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 80. | Rühl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol Motil. 2004;16 Suppl 1:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (3)] |
| 81. | Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 227] [Article Influence: 8.4] [Reference Citation Analysis (1)] |