Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4524
Revised: March 12, 2006
Accepted: March 27, 2006
Published online: July 28, 2006
AIM: To evaluate the usefulness of various computed tomography (CT) findings including distribution of infiltration or fluid collection in differentiating the major etiologies of acute pancreatitis.
METHODS: We reviewed 75 relatively severe cases of acute pancreatitis of alcoholic (n = 43) or biliary stone (n = 32) etiology having infiltration or fluid collection on CT. We compared the pancreatic size, CT grading, presence or absence of biliary calculi, and dilatation of pancreatic or bile duct. We also evaluated degree and the distribution of infiltration and fluid collection in each group.
RESULTS: The sizes of pancreas were not different between alcohol group and stone group. Alcohol group showed higher CT grading than stone group (P < 0.05). Presence of biliary stone and duct dilatation was statistically significant in differentiating etiology (P < 0.05). Alcohol group showed significantly prominent peripancreatic pathology than stone group only in left peritoneal compartment (P = 0.020).
CONCLUSION: Alcoholic pancreatitis tends to form more prominent peripancreatic changes than gallstone pancreatitis in relatively severe cases. This is evident on the anterior aspect of left abdomen. Although clinical history and some CT findings usually are a major determinant of the etiology, this pattern of peripancreatic pathology may have an ancillary role in determining the etiologies of acute pancreatitis in the equivocal cases.
- Citation: Kim YS, Kim Y, Kim SK, Rhim H. Computed tomographic differentiation between alcoholic and gallstone pancreatitis: Significance of distribution of infiltration or fluid collection. World J Gastroenterol 2006; 12(28): 4524-4528
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4524.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4524
Biliary stones and alcohol account for 70%-80% of all acute pancreatitis etiologies[1]. Differentiation between etiologies is of great importance because it can affect the further diagnostic and therapeutic strategies. Diagnosis and treatment of gallstone pancreatitis require an endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy, but it is not warranted in alcohol-induced pancreatitis[2]. The risks associated with ERCP outweigh any benefit unless the causative biliary stone is present.
Computed tomography (CT) findings associated with gallstone pancreatitis such as presence of stone in the biliary system have been well defined. Clinical information about biliary colic or alcohol abuse is also very useful in diagnosis. Causes of acute pancreatitis are often easily determined. However, some cases lacking medical background or the localization of stones or sludge make cause determination difficult. Although CT findings cannot be a sole determinant of therapy in individual cases, it can play a complementary role especially in indeterminate cases.
The purpose of this study was to evaluate the usefulness of CT findings associated with acute pancreatitis and to differentiate between the two major causes of acute pancreatitis. Special focus was given to the degree and distribution of peripancreatic infiltrations and fluid collection.
Abdominal CT scans of 86 patients who had CT diagnosis of acute pancreatitis and were evaluated to have an infiltration or fluid collection in the abdominal cavity were studied retrospectively. All of them could be graded as C, D, and E by the system suggested by Balthazar et al[3]. Grade C was defined as pancreatitis with peripancreatic infiltration only. Grades D and E were defined as the disease with single and multiple peripancreatic fluid collections, respectively. Data were not consecutive but extracted randomly from our radiology department CT database. The population was composed of 53 male patients and 33 female patients with a mean age of 56.5 (range, 20-85) years. Patients were divided into an alcohol group and a stone group based upon their clinical diagnosis made by taking into account all possible factors including past medical history such as alcohol abuse or biliary colic, results of radiological studies (ultrasonography, CT, and ERCP), and laboratory data.
Of the 86 patients, eleven were excluded from the study because of unknown causes (n = 6), causes other than alcohol abuse or biliary stone (n = 3), traumatic pancreatitis (n = 1), L-asparaginase-induced pancreatitis (n = 1), pancreatitis due to pancreatic divisum (n = 1), and cases that were indeterminable between alcoholic and stone pancreatitis (n = 2) clinically. The remaining 75 patients were composed of 49 male patients and 26 female patients, with a mean age of 51.3 (range, 27-85) years. Forty-three out of 75 patients (57.3%) were classified as alcohol group, while 32 out of 75 (42.7%) were classified as stone group.
The Institutional Review Board of our hospital did not require approval for retrospective clinical study.
Abdomen CT scans were performed with one of the two helical scanners (Somatom Plus or Somatom Plus 4; Siemens, Erlangen, Germany). Helical CT images of pre-contrast and contrast-enhanced scans were acquired using an 8-mm collimation and 10-mm/s speed (Table 1). Images were reconstructed at an 8-mm interval. A voltage of 120 kVp and amperage of 210 mAs were used. One hundred mL of non-ionic contrast material (Iopromide, Ultravist-300; Schering AG, Berlingen, Germany) was administrated intravenously via the antecubital vein at a rate of 3 mL/s with a power injector (OP 100; Medrad, Pittsburgh, PA). Dual-phase contrast-enhancement scan technique was adopted with a delay time of 60-70 s and 200-220 s, respectively, in the majority of cases. Pancreatic phase scan with a delay time of 30-40 s was available only in a small portion of the patients (n = 13) because of lacking of the clinical diagnosis of acute pancreatitis before CT examination. At least one of the two or three phases covered an entire abdominal cavity from diaphragmatic dome to symphysis pubis.
| Findings | Alcohol group (n = 43) | Stone group (n = 32) | P | |
| Size of pancreas | ||||
| Anteroposterior diameter, Head | 36.2 ± 6.7 mm | 37.3 ± 6.6 mm | 0.485 | |
| Transverse diameter, Head | 30.7 ± 5.6 mm | 31.7 ± 5.4mm | 0.434 | |
| Thickness, Body and Tail | 22.3 ± 5.2 mm | 23.3 ± 5.3 mm | 0.406 | |
| CT grading score | 2.3 ± 0.8 | 1.9 ± 1.0 | 0.0471 | |
| Calculi in the biliary system | 3/43 (7.0%) | 22/32 (68.8%) | 0.0001 | |
| Duct dilatation | ||||
| Pancreatic duct | 4/43 (9.0%) | 10/32 (31.3%) | 0.0331 | |
| Bile duct | 2/43 (4.7%) | 19/32 (59.4%) | 0.0001 | |
Two abdominal radiologists analyzed the CT findings in consensus. In patients undergoing multiple follow-up CT scans, only the initial study was selected for imaging analysis. The antero-posterior and transverse diameters of pancreatic head, as well as the thickest dimension of body or tail of pancreas were measured. We evaluated the severity of disease at the initial abdominal CT using the CT grading system introduced by Balthazar et al[3]. We classified them into a three-point-scale, and converted C, D, and E to 1, 2, and 3. We investigated the presence or absence of calculus. Calculus was thought to be present when we saw high attenuation in the biliary tree including gallbladder on pre-contrast scan without measurement of Hounsfield unit. We also examined whether the pancreatic or bile duct was dilated or not. The criteria for abnormal duct dilatation were over 8 mm in diameter for common bile duct and 3 mm in diameter for pancreatic duct.
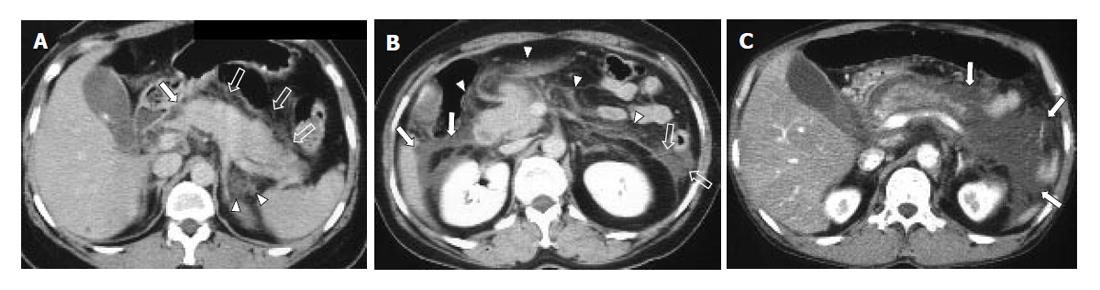
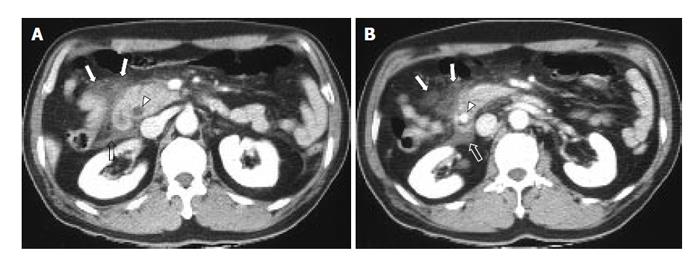
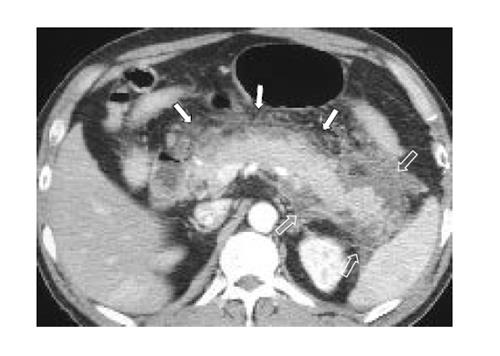
To assess the distribution of peripancreatic infiltration or fluid collection, the abdominal cavity was divided into 6 compartments, namely right peritoneal (RP) compartment, right superior retroperitoneal (RSR) compartment, right inferior retroperitoneal (RIR) compartment, left peritoneal (LP) compartment, left superior retroperitoneal (LSR) compartment, and left inferior retroperitoneal (LIR) compartment. ‘Right’ and ‘left’ compartments were divided by median line of the body traversing the umbilicus and the spinous process of vertebra. ‘Peritoneal’ and ‘retroperitoneal’ compartments were divided by already-established anatomical interface between peritoneum and retroperitoneum. Finally, ‘superior’ and ‘inferior’ compartments were compartmentalized by the level of lower pole of the left kidney. Degrees of infiltration or fluid collection in each compartment were evaluated with a four-point scale from 0 to 3 (0: no infiltration, 1: irregular infiltrative attenuation without fluid collection, 2: fluid collection with no or equivocal degree of mass effect on adjacent organ, 3: fluid collection with a definite mass effect on adjacent organ) (Figure 1). If major infiltration or fluid collection of one compartment slightly extended to another compartment, the minor lesion was disregarded. Pancreatic necrosis or pseudocyst was regarded and analyzed as a fluid collection. Infiltration scores of each abdominal compartment in one group were compared with those of the corresponding compartment of the other group. Student t-test (pancreatic size), likelihood ratio test (CT grading of acute pancreatitis, grading of infiltration and fluid collection), and Fisher’s exact test (calculi and duct dilatation) with 95% confidence interval were used for the evaluation of statistical significance (SPSS for Windows 11.0, Chicago, IL).
The antero-posterior and transverse diameters of pancreatic head for the alcohol and stone group measured 36.2 ± 6.7 mm × 30.7 ± 5.6 mm and 37.3 ± 6.6 mm × 31.7 ± 5.4 mm, respectively. The thickness of pancreatic body or tail in great dimension was 22.3 ± 5.2 mm and 23.3 ± 5.3 mm, respectively. Differences in pancreas dimension between the alcohol and stone groups were not significant (P > 0.05, Student-t test).
Scores of alcohol and stone groups resulting from CT grading (converted from C, D, E to 1, 2, 3), were 2.33 ± 0.81, and 1.94 ± 0.95, respectively. Alcohol group showed more aggressive CT findings than stone group (P = 0.047, likelihood ratio test).
The alcohol group showed calculi in the biliary tree in 3 of 43 cases (7.0%), while 22 of 32 cases (68.8%) were calculus-positive in the stone group (P = 0.000, Fisher’s
exact test). However, only 5 of 22 stone-positive cases showed calculi in the distal common bile duct. Only 5 of 32 cases (15.6%) in stone group showed distal common bile duct calculi on CT scans. In the remaining cases of stone group, biliary calculi were located in the gallbladder (n = 11), intrahepatic duct (n = 1), common duct other than distal portion (n = 3), and simultaneously in the gallbladder and the common duct other than distal portion (n = 2).
Abnormal pancreatic duct dilatation was noted in 4 of 43 cases (9.3%) of alcohol group and in 10 of 32 cases (31.3%) stone group (P = 0.033, Fisher’s exact test). Bile duct dilatation was positive in 2 of 43 cases in 19 of 32 cases (59.4%) alcohol group, 59.4% (19/32) of stone group (P = 0.000, Fisher’s exact test). Abnormal dilatation of either the pancreatic or bile duct had a statistical significance in differentiating between the two groups.
Infiltration scores of each abdominal compartment are summarized in Table 2. The overall degree of peripancreatic infiltration of right abdomen (sum of scores of RP + RSR + RIR) was almost same each other (2.40 ± 2.16) as in stone group (2.41 ± 2.12) (P = 0.798, likelihood ratio test). However, in the left abdominal compartments (LP + LSR + LIR), stone group (2.41 ± 1.79) showed a tendency of less peripancreatic infiltration than alcohol group, but not significant (3.67 ± 2.20) (P = 0.153). Among the six abdominal compartments, only the peritoneal aspect of left abdomen (or LP compartment) showed a significant difference in peripancreatic change between the two groups (1.67 ± 0.97 in alcohol group, 1.00 ± 0.92 in stone group, P = 0.020, likelihood ratio test).
| Compartment | Alcohol group (n = 43) | Stone group (n = 32) | P |
| RP | 1.00 ± 0.82 | 0.91 ± 0.69 | 0.568 |
| RSR | 0.84 ± 0.87 | 0.94 ± 0.95 | 0.416 |
| RIR | 0.56 ± 0.85 | 0.56 ± 0.84 | 0.905 |
| Total (right) | 2.40 ± 2.16 | 2.41 ± 2.12 | 0.798 |
| LP | 1.67 ± 0.97 | 1.00 ± 0.92 | 0.0201 |
| LSR | 1.49 ± 0.88 | 1.13 ± 0.83 | 0.119 |
| LIR | 0.51 ± 0.28 | 0.83 ± 0.52 | 0.240 |
| Total (left) | 3.67 ± 2.20 | 2.41 ± 1.79 | 0.153 |
The representative cases of acute pancreatitis caused by biliary stone and alcohol are presented in Figures 2 and 3, respectively.
Acute pancreatitis has numerous causes and an obscure pathogenesis. The exact pathogenetic mechanism of acute pancreatitis has not been completely established especially in the field of alcohol-induced pancreatic injury. The basic pathogenesis of acute pancreatitis is pancreatic autodigestion. Premature activation of zymogens within acinar cells, escape of activated enzymes from acinar cells and pancreatic ducts start the autodigestive process. It has not been established how alcohol abuse induces premature zymogen activation and release. However, the mechanism of gallstone pancreatitis is known via animal models. A stone impacted in the ampulla of Vater raises intraductal pressure. Increased pressure makes the pancreatic duct epithelium permeable to molecules of up to 25 000 Da. Acute pancreatitis occurs when the pancreatic duct is perfused with active pancreatic enzymes, particularly when microvascular permeability is increased by the actions of histamine or prostaglandins. Thus, pancreatic zymogen activation and increased pancreatic duct permeability may act sequentially initiating acute pancreatitis[1,4].
Despite this background knowledge of the pathogenesis of acute pancreatitis, we cannot readily explain why alcohol-induced pancreatitis forms more peripancreatic infiltration or fluid collection than gallstone pancreatitis, especially on the anterior aspect of the left-sided abdomen. As a candidate of the possible explanation for this phenomenon, we assume that the state of intoxication as well as the pain-killing effect of alcohol may play a role in masking acute symptoms of pancreatitis and make patients delay to be hospitalized. On the contrary, biliary stone itself often causes severe pain even before the initiation of acute pancreatitis. However, we cannot explain more prominent involvement of the anterior aspect of the left-sided abdomen by alcohol-induced pancreatitis. This tendency of a more aggressive form of pancreatitis by alcohol-induced disease than stone-associated disease is supported by some previous studies[5,6].
Most previous efforts to differentiate alcoholic and non-alcoholic pancreatitis have been based on laboratory data or clinical manifestations[7-10], while there are few studies using imaging modality. The authors of these studies have tried at best, to visualize a stone or bile sludge in the biliary system[11].
There was a prominent discrepancy between the rate of visible common bile duct stone on pre-contrast CT scan (15.6%) and the rate of common bile duct dilatation (59.4%) in our study. We think that this discrepancy is due to (1) a passed stone with post-inflammatory swelling of ampula of Vater, (2) a muddy stone in the common bile duct that could not be differentiated from bile on CT scan without measurement of Hounsfield unit, or (3) pancreatic swelling because of acute pancreatitis itself.
Only 15.6% of patients with gallstone pancreatitis showed calculi impacted in the ampulla of Vater although 68.8% were positive for gallstone pancreatitis if the entire biliary tree was included. In other words, 31.2% of gall stone pancreatitis patients did not show any calculi in the biliary system on abdominal CT scans. In most of these cases, diagnosis of gallstone pancreatitis was achieved through other imaging modalities such as ultrasonography or ERCP. Still in a small number of cases, serum bilirubin tests and/or patient history were needed for their clinical diagnosis. Similar situations are not uncommon in daily practice making ancillary CT findings meaningful in the clinical determination of acute pancreatitis etiology.
Based on our analysis, we assumed that the extent of the infiltration or fluid collections formed by acute pancreatitis could reflect the severity of the disease, as described by Balthazar et al[5]. Generally, infiltrations or fluid collections of acute pancreatitis tend to involve retroperitoneum rather than peritoneum because the pancreas is located in the retroperitoneal space[12]. On this point of view, we divided the retroperitoneum into superior and inferior compartments, and we also assumed that, initially, infiltrations might extend to retroperitoneum around pancreas (or superior compartment of retroperitoneum), then simultaneously further inferiorly along the retroperitoneal space and anteriorly to peritoneal space. Therefore, it is necessary to compartmentalize retroperitoneum into superior and inferior and score each compartment independently to weight on the significance of retroperitoneal space as an initially-preferred pathway of disease spread.
Population of our study was confined to the patients who had peripancreatic pathologic findings at initial abdominal CT. Therefore, results from our study cannot be applied to all patients with acute pancreatitis, but should be restricted to the moderate or severe cases in which CT shows peripancreatic pathology. However, we have met relatively severe forms of acute pancreatitis much more frequently in daily CT practice, and these are the real cases needing differentiation of their etiologies. So, we think the results from our study could be helpful.
Pancreatic necrosis is undoubtedly a very important factor for poor prognosis of acute pancreatitis. It has been proven that patients with necrotizing pancreatitis have much a higher mortality and complication rate than patients with simple pancreatitis[13]. We regard a pancreatic necrosis as a mere fluid collection because we just want to know the meaning of the extent and distribution of fluid collection in differentiating major etiologies of acute pancreatitis. Whether the fluid is a necrotic pancreatic parenchyma or just a fluid remains unclear. However, we cannot agree that it is a potential limitation of our study.
Other limitations of our study are as follows, (1) Selection of patients was randomized but it was retrospective and not consecutive, possibly introducing potential selection bias; (2) The gold standard grouping of alcoholic and gallstone pancreatitis was the clinical diagnosis using medical records. Improper clinical history taking might play a role in incorrect determination of etiology of acute pancreatitis.
In conclusion, alcoholic pancreatitis tends to form more peripancreatic infiltrations or fluid collections than gallstone pancreatitis. This tendency is more prominent on the anterior (peritoneal) aspect of the left abdomen. Although clinical histories such as biliary colic or alcohol abuse and some CT findings favoring a gallstone pancreatitis usually are a major determinant of the etiology of acute pancreatitis, the degree and distribution of peripancreatic infiltration or fluid collection may have an ancillary role in differentiating the two major etiologies of acute pancreatitis especially in the case of insufficient clinical history or passed-out stone.
| 1. | Soergel KH. Cecil Textbook of Medicine. 20th ed. Philadelphia: W.B. Saunders 1996; 729-736. |
| 2. | Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 470] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-772. [PubMed] |
| 4. | Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 5. | Pezzilli R, Billi P, Morselli-Labate AM. Severity of acute pancreatitis: relationship with etiology, sex and age. Hepatogastroenterology. 1998;45:1859-1864. [PubMed] |
| 6. | Lankisch PG, Assmus C, Pflichthofer D, Struckmann K, Lehnick D. Which etiology causes the most severe acute pancreatitis. Int J Pancreatol. 1999;26:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Gumaste VV, Dave PB, Weissman D, Messer J. Lipase/amylase ratio. A new index that distinguishes acute episodes of alcoholic from nonalcoholic acute pancreatitis. Gastroenterology. 1991;101:1361-1366. [PubMed] |
| 8. | Tenner SM, Steinberg W. The admission serum lipase: amylase ratio differentiates alcoholic from nonalcoholic acute pancreatitis. Am J Gastroenterol. 1992;87:1755-1758. [PubMed] |
| 9. | Stimac D, Lenac T, Marusic Z. A scoring system for early differentiation of the etiology of acute pancreatitis. Scand J Gastroenterol. 1998;33:209-211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ammori BJ, Boreham B, Lewis P, Roberts SA. The biochemical detection of biliary etiology of acute pancreatitis on admission: a revisit in the modern era of biliary imaging. Pancreas. 2003;26:e32-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Schölmerich J, Johannesson T, Brobmann G, Wimmer B, Thiedemann B, Gross V, Gerok W, Farthmann EH. [Sonography in acute pancreatitis--diagnosis, assessment of etiology and evaluating prognosis]. Ultraschall Med. 1989;10:290-294. [PubMed] |
| 12. | Siegelman SS, Copeland BE, Saba GP, Cameron JL, Sanders RC, Zerhouni EA. CT of fluid collections associated with pancreatitis. AJR Am J Roentgenol. 1980;134:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 116] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Wang XL E- Editor Bi L