Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4517
Revised: May 12, 2005
Accepted: August 3, 2005
Published online: July 28, 2006
AIM: To study the esophageal geometry and mechanosensation using endoscopic ultrasonography during volume-controlled ramp distensions in the distal esophagus.
METHODS: Twelve healthy volunteers underwent distension of a bag. During distension up to moderate pain the sensory intensity was assessed on a visual analogue scale (VAS). The esophageal deformation in terms of multidimensional stretch ratios and strains was calculated at different volumes and VAS levels. Distensions were done before and during administration of the anti-cholinergic drug butylscopolamine.
RESULTS: The stimulus-response (volume-VAS) curve did not differ without or with the administration of butylscopolamine. Analysis of stretch ratios demonstrated tensile stretch in circumferential direction, compression in radial direction and a small tensile stretch in longitudinal direction. A strain gradient existed throughout the esophageal wall with the largest circumferential deformation at the mucosal surface. The sensation intensity increased exponentially as function of the strains.
CONCLUSION: The method provides information of esophageal deformation gradients that correlate to the sensation intensity. Hence, it can be used to study mechanosensation in the human esophagus. Further studies are needed to determine the exact deformation stimulus for the esophageal mechanoreceptors.
- Citation: Larsen E, Reddy H, Drewes AM, Arendt-Nielsen L, Gregersen H. Ultrasonographic study of mechanosensory properties in human esophagus during mechanical distension. World J Gastroenterol 2006; 12(28): 4517-4523
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4517
Acute and chronic pain in the upper gastrointestinal tract is a severe and frequent health care problem in terms of human suffering and enormous economic implications for the health care system[1]. Thorough understandings of visceral pain mechanisms are necessary for developing diagnostic tools and optimal treatments. However, pain and related symptoms are difficult to study due to the heterogeneity of patients and confounders related to clinical pain[2].
Human experimental pain models can be used as tools for better characterization and understanding of pain mechanisms. In recent years studies have been focused on mechanical distension[3-5]. However, many experimental pain studies of hollow visceral organs have reached erroneous conclusions since the pressure, volume and wall tension are proxies of the deformation in the vicinity of mechanoreceptors and therefore only show indirect degree of association with the evoked responses. It has been demonstrated in several parts of the digestive tract including the esophagus that deformation (circumferential strain) is more closely associated with the distension-induced sensory responses than the distension pressure, volume or wall tension[6-8]. Calculation of the strain, however, demands advanced methods. Recently, endoscopic ultrasonography has been used to obtain information about the geometry of the esophagus including wall and layer thicknesses for the purpose of computing wall tension, stress and strain during swallowing[9-12]. However, none of these studies has carefully evaluated the sensory properties during esophageal distension.
In the present study, endoscopic ultrasonography was combined with controlled esophageal distension and sensory assessment to obtain information about the mechanosensation. Since deformation is considered the most important parameter for mechanosensation[6-8], we measured the needed geometric variables and computed multidimensional stretch ratios and strains in order to obtain new information about the relationship between stretch and evoked pain.
Twelve healthy subjects, 6 males and 6 females, median age 38 years (range 22-67 years) recruited from the hospital and university staff, volunteered to participate in the study. They did not suffer from previous or current gastrointestinal disease and had no gastrointestinal complaints. The volunteers did not take any drugs and abuse alcohol. The regional ethics committee approved the study protocol and the experiments were conducted in accordance with the revised Declaration of Helsinki. All volunteers were asked to give a written informed consent to participate in the experiment.
The probe was constructed from a thin flexible tube and equipped at the end with a highly compliant latex balloon. The tube was connected to an infusion pump (Type 111, Ole Dich Instrument makers, Hvidovre, Denmark). Saline heated to body temperature was infused into the balloon at the rate of 25 mL per min. The 6-cm long balloon was made of natural rubber with a thickness of 0.2 mm. In the deflated state the largest transversal diameter of the balloon was 10 mm. The shape of the balloon became almost spherical when inflated. An ultrasonographic miniprobe was advanced through the probe lumen and the transducer was placed inside the balloon. The ultrasound system used a rotating 20-MHz transducer (model UM-3R; Olympus, Japan). The ultrasound transducer produced a real time 360-degree cross-sectional image of the esophagus.
The subjects were fasted at least for 6 h. Studies were performed with the subjects in half supine position. The probe was lubricated and inserted through the nostrils. The tip of the probe was first advanced into the stomach. The lower esophageal sphincter (LES) was identified by pull-through manometry as a zone of resting pressure that decreases with swallowing. The balloon was placed 8 cm proximal to LES and the probe was fixed to the nose to prevent migration of the assembly during the study.
Distensions were done to precondition the tissue and served as learning sessions to facilitate the sensory rating. Since preconditioning is necessary to obtain reproducible results[4], three distensions using a constant infusion rate of 25 mL/min were done until the pain threshold was reached (5 on the visual analogue scale (VAS). After the preconditioning distensions, two more distensions to 7 on the VAS (moderate pain level) were done. Then a bolus of 20 mg butylscopolamine was given intravenously to decrease distension-induced secondary smooth muscle contractions. The balloon was again inflated twice using an infusion rate of 25 mL/min until 7 on the VAS was reached. The balloon was emptied for 2 min between the distensions. None of the volunteers experienced significant side effects due to the anti-cholinergic action of the drug. The total investigation time never exceeded 1.5 h.
The sensory intensity was assessed continuously during the mechanical distension by using an electronic VAS-meter (GMC-Medical, Hornslet, Denmark). The VAS data were amplified and analog-to-digital converted at a sampling rate of 10 Hz using the Openlab data acquisition system (GMC-Medical, Hornslet, Denmark). The digitized data were stored on a PC for later analysis.
Before commencement of the visceral mechanical stimulation, the subjects were trained in using the VAS. The sensations were produced by applying deep pressure in muscles of the right forearm. The intensity of non-painful stimuli was scored up to 5 on the VAS. Key anchor words to describe the sensations were: 1 = vague perception of mild sensation; 2 = definite perception of mild sensation; 3 = vague perception of moderate sensation; 4 = definite perception of moderate sensation; 5 = pain threshold. For the painful sensations the patients used the scale from 5-7 anchored at 6 = slight pain and 7 = moderate pain[2].
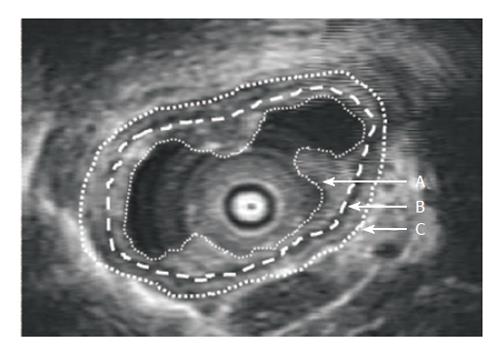
Ultrasonographic images of the esophagus were recorded in real time during the studies using a super-VHS videotape recorder. The videotape recordings were captured in video streaming 120 + MB/s at a frame rate of 25 s-1 and a resolution 756 × 576 by a high performance video grabber (I-Color, Image House A/S Copenhagen DK). All image measurements and calculations were performed off-line using an image analysis software package (Scion Image Beta 4.02 Win, Maryland). We selected one reference image at each pain intensity level from the distensions after preconditioning the tissue. The images were selected at points where contractions were not present. The mucosa and muscle layers were outlined manually (Figure 1). Once outlined, the software program automatically calculated the circumference and the area of the layers. The layer thickness was computed from the circumferential length and area.
The steady state pressure, diameters, CSA and wall thickness values at each volume distension step were used for the computation of stretch ratios and strains.
The circumferential Cauchy strain ε was computed as the fractional change in circumference[3]. ε=(c-c0)/c0, where C is the circumference obtained at the serosal or mucosal surfaces or between layers at various degrees of distension, C0 is the reference circumference obtained at unloaded conditions immediately prior to a distension. The strain in radial direction could be computed from the measured wall thickness h as ε=(h-h0)/h0, where h0 is the reference thickness obtained at unloaded conditions immediately prior to a distension. In a similar way the strain could be computed for the individual wall layers.
In a mechanical analysis it may also be convenient to use the stretch ratio as a measure of deformation. The stretch ratio λ was determined for both the circumferential (θ), radial (r) and longitudinal (z) direction. The stretch ratio is defined as the length at loaded conditions (L) divided by a reference length at unloaded conditions (L0). λ=L/L0, thus the stretch ratio λ=ε+1. For circumferential and radial direction the stretch ratios could be determined from the experiments using the same principle as for the strain calculation. The stretch ratio in longitudinal direction λz was determined from the two other stretch ratios by using the law of mass conservation. Hence, λz = 1/λθλr [3,4,13].
The results were expressed as mean ± SE. At various volumes and VAS levels 1-7, the corresponding stretch ratios before and during administration of butylscopolamine were determined. Two-way-ANOVA was used for statistics. P < 0.05 was considered statistically significant.
Ultrasonographic and VAS data were obtained in all subjects. The distensions were done until moderate pain (VAS = 7) was experienced except in one subject in whom the distension was done only until the pain threshold (VAS = 5) was reached. Three subjects repeatedly got involuntary long lasting contractions in the esophagus after the pain threshold was reached. Butylscopolamine greatly reduced these contractions. One subject reported autonomic side-effects during the distensions manifested as tachycardia, nausea and sweating.
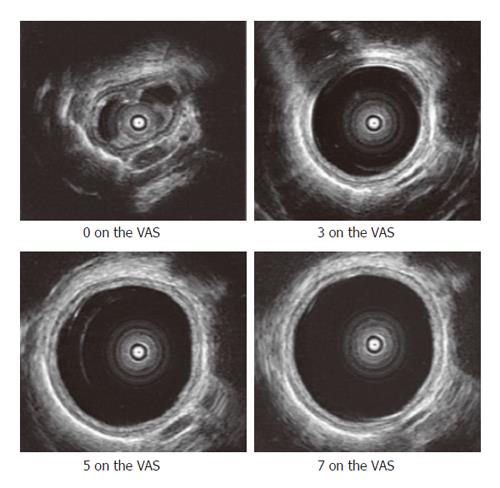
The preconditioning stimuli resulted in reproducible mechanical distensions and sensory-motor responses. Figure 2 shows the representative ultrasound images obtained at different VAS levels. The mean thickness of the mucosa and muscle layer in the resting state was 1.4 ± 0.02 mm and 2.4 ± 0.03 mm respectively. The esophageal folds almost disappeared at VAS level of 3 or higher where the geometry could be approximated as circular. The interface between the inner circular and outer longitudinal muscular layer could be visualized as a thin echogenic layer only at low volumes in the balloon. This layer was not consistently visible in the subjects at VAS levels of 2 or higher (Figure 2) and therefore could not be used in the strain analysis.
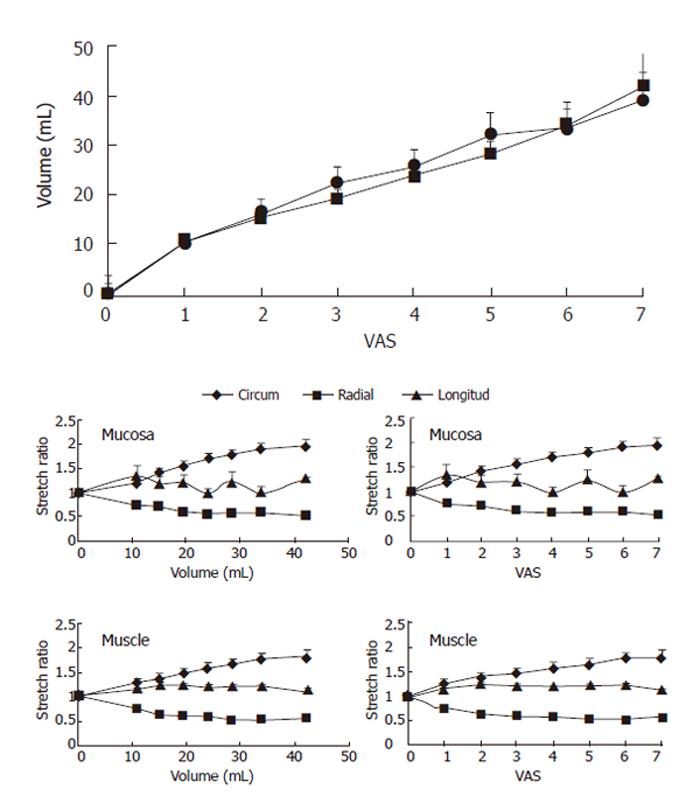
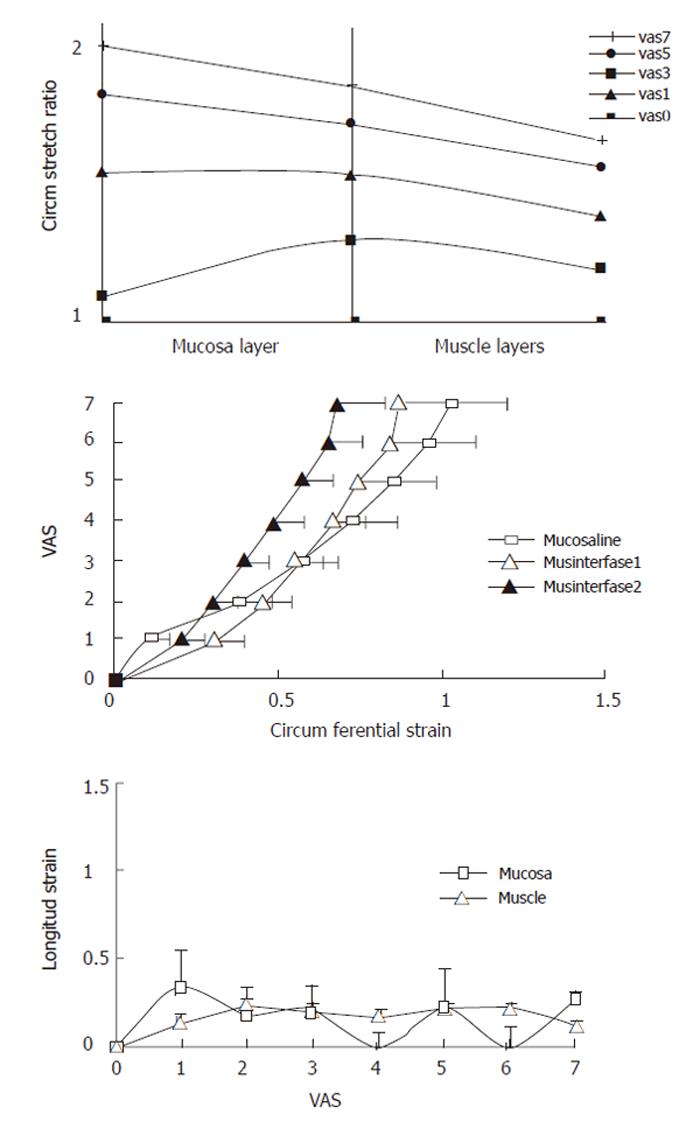
We found no difference in the volume-VAS data before and during butylscopolamine infusion (Figure 3-top, F = 2.50, P = 0.12). Consequently we used the data obtained during butylscopolamine infusion for the further analysis since contractions to some degree would bias the readings. Thus, the increased VAS primarily would be due to stretch of the wall rather than the active stress generated by the muscle tissue. Figure 3 also shows the circumferential, radial and longitudinal stretch ratios as function of volume and sensory intensity on the VAS for the mucosa and main muscle layers. In both layers, the circumferential deformation was tensile, whereas the radial deformation was compressive due to the decrease in layer thickness during the distensions. The computed longitudinal stretch ratio was tensile but less than the circumferential stretch ratio. Furthermore, the longitudinal stretch ratio did not increase further after 1 was reported on the VAS or a volume of 10 mL was reached. At the pain threshold (VAS 5) the mean circumferential, radial and longitudinal stretch ratios in the muscle layer were 1.7 ± 0.1, 0.5 ± 0.04 and 1.2 ± 0.07, respectively.
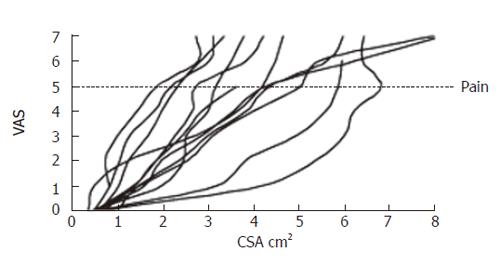
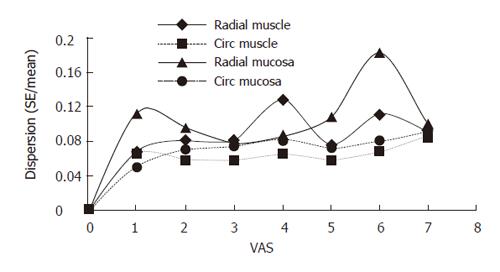
The sensory response showed a large variation between the volunteers. Figure 4 shows the sensation as function of luminal cross-sectional area (CSA). The rather large inter-individual variation speaks in favor of using non-dimensional measures such as stretch ratios rather than the volume or CSA. Figure 5 shows the circumferential stretch level at the mucosa and the inner and outer muscle layers. The circumferential deformation at the mucosal surface was larger than that at the outer muscle layer at high sensory levels. However, at low VAS levels the mucosal deformation was small, which was due to unfolding rather than stretch of the mucosa initially during the distensions. A strain gradient existed through the esophageal wall with the largest deformation in the mucosa and the sensation increased exponentially as function of the strain in both the mucosa and muscle layers (Figure 5). Longitudinal strain during distension seemed not to cause the sensation as the longitudinal strain did not increase further after VAS 1 (Figure 5 bottom). To evaluate further whether radial or circumferential strains were most important, we plotted the dispersion (SE/mean) of the radial and circumferential stretch ratio data from Figure 3. The circumferential stretch ratios in the muscle and mucosa layers showed the smallest dispersion (Figure 6).
The function of the esophagus is mechanical to a large extent. Swallowed contents are propelled through the esophagus into the stomach where the digestion starts. Methods traditionally used for clinical or basic investigations of the esophagus are endoscopy, manometry, pH-metry and radiographic examinations. Although these methods provide important data on esophageal motor function, little attention has been paid to the biomechanical and mechanosensory properties of the wall and determination of the wall deformation. During recent years ultrasonography has gained interest in studying gastrointestinal (GI) physiology and in clinical evaluation of the esophagus. In the current study, the stretch was tensile in circumferential direction, compressive in radial direction and tensile to a small degree in the longitudinal direction. A strain gradient existed throughout the esophageal wall with the largest circumferential deformation at the mucosal surface. The sensation intensity increased exponentially as function of the strains and the esophageal deformation gradients correlated to the sensation intensity.
Endoscopic ultrasonography is a non-invasive and safe method applicable in study of the esophagus. Two-dimensional B-mode ultrasonography has been utilized to assess the correlation between ultrasonographic images and histology[13] and to determine biomechanical properties of the esophagus in vitro[14]. Furthermore, Nicosia et al[10] have used endoscopic ultrasonography in humans to study esophageal longitudinal muscle shortening and Miller et al[11] have studied the correlation between wall changes and manometry during esophageal motility. Moreover, Pehlivanov et al[9] showed that muscle geometry is changed during contractions in volunteers and patients with esophageal spasms. None of these studies has paid attention to the sensation provoked by balloon distension. Thus, despite these developments and the fact that the mechanical wall properties are important for normal function of the esophagus, the mechanosensory properties of the esophagus in vivo are still largely unknown. In this study we therefore intended to gain a better understanding of esophageal deformation and mechanosensory function.
In distensible biological tubes, the circumferential wall strain is of interest because the tensile stress is the largest in that direction during distension[3,4,15]. The reasons for studying the stresses and strains in intact hollow organs are as follows: wall stress and strain data give valuable information on the elastic properties[4], mechanoreceptors do not respond directly to changes in pressure or volume but rather to changes in strain[2], it is important to differentiate active properties such as phasic contractions and smooth muscle tone from passive tissue properties in pharmacological and biomechanical studies[2,16,17], and intact organs maintain normal geometry in contrast to the tissue strips often used for length-tension measurements in physiological and pharmacological studies[15,18].
In this study, we aimed to develop a new method for investigation of the mechanosensory properties in the esophagus, mainly in the state where contractions did not confound the analysis. The method is based on distension of a bag placed in the lumen with concomitant measurement of the distension pressure and the antral geometry in a selected cross-sectional plane by endoscopic real-time B-mode ultrasound. The ramp protocol ensured that we studied the elastic properties rather than viscoelastic properties[4,15]. This ease the interpretation of the data since time-dependent confounders are significantly eliminated[4]. The administration of the antimuscarinic drug butylscopolamine allowed us to investigate the active and passive tissue behavior. Since the volume-VAS curve did not differ between the distensions before and during butylscopolamine administration, we only analyzed the distensions after administration of butylscopolamine. This gives data that are easier to interpret as there is no significant influence of esophageal contractions. Butylscopolamine may not abolish all contractions. Therefore we analyzed the data between contractions if they were present. Since stretch as well as contractions may elicit pain, we primarily looked into the stretch-dependent pain mechanisms in this study. It has been found in other tissues as in the cardiovascular system that the baroreceptors depend on strains in the vicinity of the receptors[15]. We have previously shown that strain is the most important biomechanical parameter for sensory responses in the gastrointestinal tract including the esophagus[6-8]. Thus, in this study we were only concerned with the deformation as a stimulus for the mechanoreceptors.
The ultrasonographic technique makes the derivation of strains in multidimensional directions possible from the geometric data or due to use of the mass conservation principle. The strain calculations are based on direct measurements rather than on geometric assumptions such as a circular cross-section and the thickness of the layers measured in our study, which are consistent with the data presented by others[10]. The fact that the lumen is not circular at low volumes makes it difficult to compute tension and stress based on Laplace’s law[3,4,15], but the strain analysis does not depend on the geometry as long as the surfaces and interfaces between layers can be clearly defined. In most cases we did not have any problems in identifying the tissue layers. Thus the error due to these measurements is considered small. However, the ultrasound images only show the primary convolution (folds) but not secondary and tertiary convolutions of the mucosa. If higher-order convolutions were present, this could result in a minor underestimation of the calculated stretch in the mucosa and a larger inter-individual variation. Unfortunately, we could not clearly outline the interface between the two muscle layers at VAS levels above 3. Hence, we omitted presenting data on this interface.
In the present study, the deformation was the largest in the circumferential direction, despite the presence of mucosal folds the circumferential deformation decreased through the wall with the largest values at the mucosal surface, and the sensation increased exponentially as function of the circumferential strain.
Gastrointestinal symptoms are often associated with disturbances in motility and sensory function. Several studies have attempted to investigate these properties by means of balloon distension[19-21]. Unfortunately, the primary mechanism for symptoms elicited by GI distension remains unclear. It is well known that distension of the gastrointestinal tract elicits reflex-mediated inhibition and stimulation of motility via intrinsic or extrinsic neural circuits and induces visceral perceptions such as pain[21]. Previous studies demonstrated that mechanoreceptors located in the intestinal wall play an important role in the stimulus-response function[22-26]. It is, however, a common mistake to believe that mechanoreceptors are sensitive to variation in pressure, volume or tension. A large variation in the perception has been found in various studies and species[27-29], suggesting that pressure is not the direct stimulus. The dogma that the mechanoreceptors are tension-sensitive receptors lying in-series or in-parallel with the muscle cells has also been prevalent for many years[30]. This concept is borrowed from striated muscle physiologists and should yet be regarded as a working hypothesis, since no clear evidence supports it in GI studies. It is basically a uniaxial model that does not account for more complex biomechanical properties such as distribution of the deformation field and that different receptor populations may exist. There seems to be no evidence that the receptor is dependent of tension rather than strain. Instead, the receptors are stimulated by deformations acting on the intestinal wall due to changes in the transmural pressure[31]. Thus, the mechanical distension stimulus and the biomechanical tissue properties must be taken into account in studies of the sensory-motor function in the esophagus.
It is well known that the passive elastic behavior of biological tissues is exponential[3,4,15]. This mechanical feature protects the tissue against over-distension and damage at high luminal pressure loads while distending easily and facilitating flow in the physiological pressure range. In arteries, it has been demonstrated that collagen bears circumferential loads at high stress levels[32,33]. As gastrointestinal tissue is rich in collagen[34], it is likely that collagen is a major determinant of the passive mechanical properties. Though we did not evaluate the elastic properties in terms of stress-strain relations, the fact that the strain-VAS curve is exponential indicates that the exponential properties play a role in protecting tissue against high stress.
Our results may shed some light on the discussion regarding the visceral sensation receptors in human beings. The data suggest that at low loads the deformation occurs mainly in the muscle layer, whereas at higher loads, the largest deformation occurs at the mucosal surface. Furthermore, the lowest dispersion has been found for the circumferential stretch ratio in the muscle layer. Though this analysis may be biased by measurement of errors for the two directions, the analysis may reveal that the most important mechanoreceptors are located in the muscle layer where they sense circumferential deformation. This view is also supported by other data showing that the circumferential deformation in the mucosa is much smaller than in the muscle layer at low loads, where the mucosa merely unfolds rather than stretches. However, the differences are small because the deformations in various directions depend on each other (due to the mass conservation principle) and require further analysis.
We have developed a new method to study the strain-dependency of esophageal mechanoreceptors. The data point in the direction of a strain receptor sensitive to circumferential deformation in the muscle layer. The new test can be used to test drugs on esophageal mechanosensory function, and improve our understanding of the pathophysiology underlying the symptom induction in patients with pain and other symptoms relating to the esophagus.
| 1. | Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting--a population-based study. Aliment Pharmacol Ther. 2003;17:1115-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: a reappraisal of human studies. Scand J Gastroenterol. 2003;38:1115-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Motil. 1996;8:277-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 206] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Gregersen H. Biomechanics of the gastrointestinal tract. London: Springer Verlag 2002; . |
| 5. | Drewes AM, Pedersen J, Liu W, Arendt-Nielsen L, Gregersen H. Controlled mechanical distension of the human oesophagus: sensory and biomechanical findings. Scand J Gastroenterol. 2003;38:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Barlow JD, Gregersen H, Thompson DG. Identification of the biomechanical factors associated with the perception of distension in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2002;282:G683-G689. [PubMed] |
| 7. | Gao C, Arendt-Nielsen L, Liu W, Petersen P, Drewes AM, Gregersen H. Sensory and biomechanical responses to ramp-controlled distension of the human duodenum. Am J Physiol Gastrointest Liver Physiol. 2003;284:G461-G471. [PubMed] |
| 8. | Petersen P, Gao C, Arendt-Nielsen L, Gregersen H, Drewes AM. Pain intensity and biomechanical responses during ramp-controlled distension of the human rectum. Dig Dis Sci. 2003;48:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Pehlivanov N, Liu J, Kassab GS, Beaumont C, Mittal RK. Relationship between esophageal muscle thickness and intraluminal pressure in patients with esophageal spasm. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1016-G1023. [PubMed] |
| 10. | Nicosia MA, Brasseur JG, Liu JB, Miller LS. Local longitudinal muscle shortening of the human esophagus from high-frequency ultrasonography. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1022-G1033. [PubMed] |
| 11. | Miller LS, Liu JB, Colizzo FP, Ter H, Marzano J, Barbarevech C, Helwig K, Leung L, Goldberg BB, Hedwig K [corrected to Helwig K. Correlation of high-frequency esophageal ultrasonography and manometry in the study of esophageal motility. Gastroenterology. 1995;109:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Takeda T, Kassab G, Liu J, Nabae T, Mittal RK. Effect of atropine on the biomechanical properties of the oesophageal wall in humans. J Physiol. 2003;547:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wiersema MJ, Wiersema LM. High-resolution 25-megahertz ultrasonography of the gastrointestinal wall: histologic correlates. Gastrointest Endosc. 1993;39:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Assentoft JE, Gregersen H, O'Brien WD Jr. Determination of biomechanical properties in guinea pig esophagus by means of high frequency ultrasound and impedance planimetry. Dig Dis Sci. 2000;45:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Fung YC. Biomechanics. Mechanical properties of living tissues. New York: Springer Verlag 1993; . |
| 16. | Gregersen H, Barlow J, Thompson D. Development of a computer-controlled tensiometer for real-time measurements of tension in tubular organs. Neurogastroenterol Motil. 1999;11:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Gregersen H, Christensen J. Gastrointestinal tone. Neurogastroenterol Motil. 2000;12:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Tøttrup A, Forman A, Uldbjerg N, Funch-Jensen P, Andersson KE. Mechanical properties of isolated human esophageal smooth muscle. Am J Physiol. 1990;258:G338-G343. [PubMed] |
| 19. | Distrutti E, Azpiroz F, Soldevilla A, Malagelada JR. Gastric wall tension determines perception of gastric distention. Gastroenterology. 1999;116:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Iovino P, Azpiroz F, Domingo E, Malagelada JR. The sympathetic nervous system modulates perception and reflex responses to gut distention in humans. Gastroenterology. 1995;108:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Serra J, Azpiroz F, Malagelada JR. Perception and reflex responses to intestinal distention in humans are modified by simultaneous or previous stimulation. Gastroenterology. 1995;109:1742-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Jorgensen CS, Dall FH, Storkholm J, Jensen SL, Gregersen H. Elastic properties of the isolated perfused porcine duodenum. Dig Dis. 1991;9:401-407. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Hukuhara T, Fukuda H. The motility of the isolated guinea pig small intestine. Jpn J Physiol. 1965;15:125-139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Hukuhara T, Nakayama S, Nanba R. Locality of receptors concerned with the intestino-intestinal extrinisic and intestinal muscular intrinsic reflexes. Jpn J Physiol. 1960;10:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 25. | IGGO A. Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q J Exp Physiol Cogn Med Sci. 1957;42:130-143. [PubMed] |
| 26. | Yokoyama S, Ozaki T. Effects of gut distension on Auerbach's plexus and intestinal muscle. Jpn J Physiol. 1980;30:143-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Akervall S, Fasth S, Nordgren S, Oresland T, Hulten L. Rectal reservoir and sensory function studied by graded isobaric distension in normal man. Gut. 1989;30:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Gregersen H, Orvar K, Christensen J. Biomechanical properties of duodenal wall and duodenal tone during phase I and phase II of the MMC. Am J Physiol. 1992;263:G795-G801. [PubMed] |
| 29. | Williams D, Thompson DG, Heggie L, Bancewicz J. Responses of the human esophagus to experimental intraluminal distension. Am J Physiol. 1993;265:G196-G203. [PubMed] |
| 30. | Tack J, Sifrim D. A little rest and relaxation. Gut. 2000;47:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ginzel KH. Investigations concerning the initiation of the peristaltic reflex in the guinea-pig ileum. J Physiol. 1959;148:75-76. |
| 32. | Dobrin PB. Mechanical properties of arterises. Physiol Rev. 1978;58:397-460. [PubMed] |
| 33. | Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol. 1957;35:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 285] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 34. | Gabella G. Structure of muscles and nerves in the gastrointestinal tract. Physiology of the gastrointestinal tract. New York: Raven Press 1987; 335-382. |
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH