Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3854
Revised: April 6, 2006
Accepted: April 14, 2006
Published online: June 28, 2006
AIM: To explore the expression of macrophage inflammatory protein-1α (MIP-1α) in Kupffer cells (KCs) following liver ischemia/reperfusion injury IRI in rats.
METHODS: Forty male SD rats were divided randomly into five groups. A model of partial warm ischemia/reperfusion injury in the rat liver was established. KCs were isolated and incubated one hour, six hours, 12 h, and 24 h after the reperfusion. Tumor necrosis factor alpha (TNF-α) and interleukin-1beta (IL-1β) in the supernatants were measured by ELISA. MIP-1α in KCs was detected by immunocytochemical and RT-PCR.
RESULTS: No or few MIP-1α protein and mRNA were expressed in the KCs of the control group. Its expression in the IRI group had a significant increase after the reperfusion (P < 0.05), which was contrary to the control group.
CONCLUSION: The active behavior of the MIP-1α gene in KCs following liver ischemia/reperfusion injury is assumed to be one of the major causes for the hepatic ischemia/reperfusion injury.
- Citation: Ma W, Wang ZR, Shi L, Yuan Y. Expression of macrophage inflammatory protein-1α in Kupffer cells following liver ischemia or reperfusion injury in rats. World J Gastroenterol 2006; 12(24): 3854-3858
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3854.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3854
Hepatic ischemia/reperfusion injury (IRI) is a common pathological process of traumatic surgical diseases in the liver, such as severe liver trauma, extensive hepatic lobus excision, liver transplantation, shock and infection[1]. It can cause a series of injuries on metabolism, structure, and function in hepatic tissues and cells, and even liver function failure. It is also one of the major factors influencing the prognosis, operative success and survival of patients. The mechanisms of liver injury caused by ischemia/reperfusion resemble those observed in other organs that are transiently deprived of oxygen. These mechanisms involve a series of events, including Kupffer cell (KCs) activation, cytokine release, neutrophil activation, increased expression of adhesion molecules, sinusoidal endothelial cell death, and hepatocyte injury[2]. Among these, the activation and the release of the excessive quantities of cytokine in the Kupffer cells play a major role.
Chemokines are members of a large and expanding family of proteins that interfere with normal (leukocyte homing, hematopoiesis, angiogenesis) and pathological (inflammation, HIV-1 infection, atherosclerosis) processes[3-7]. Depending on the positioning of the cysteine residues, chemokines are classified as C, CC, CXC and CX3C chemokines. MIP-1α is from the CC (cysteinecysteine) chemokine subfamily. These soluble factors are chemotactic for specific types of leukocyte populations and are involved in the regulation of cell-mediated immunity[8]. MIP-1α is produced by monocytes, macrophages, lymphocytes, and other cell types[9]. CCR1, CCR4, and CCR5 are the known receptors that bind to MIP-1α[10]. In addition, it has been demonstrated that these CC chemokines can induce mononuclear macrophage to secrete TNF-α and IL-1β[11]. For all the significant roles that these CC chemokines play in immune and inflammatory responses, their role in the mechanism of hepatic ischemia/reperfusion injury is not well understood. Therefore, we established a model of rat hepatic ischemia/reperfusion injury at the room temperature, isolated the Kupffer cells at different times, that is, 0 h, 1 h, 6 h, 12 h and 24 h after the onset of reperfusion, to explore the expressions of MIP-1α protein and mRNA in the Kupffer cells following liver ischemia/reperfusion injury.
Type IV collagenas and percoll were purchased from Sigma, Inc, (USA). Triozol was from Invitrogen, Inc, (USA). RNA PCR Kit and DNA Maker were purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. (China). Lysozyme, MIP-1α polyclonal antibody and SABC kit were purchased from Wuhan Borsd Biological Engineering Co., Ltd. (China). TNF-α and IL-1β ELISA kit were obtained from Shanghai Sengxiong Biotech Industry Co., Ltd. (China).
Male Sprague Dawley (SD) rats weighing 250-300 g were used in the study. They were obtained from the Experimental Animal Center of the Xi’an Jiaotong University. All animals were housed in a macroion cage in rooms maintained at a temperature of 22-24°C using a 12/12-h light/dark cycle. The animals were given a standard rat chow and fasted overnight before the experiment with water allowed ad libitum. Care was provided in accordance with the procedure outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication No.85-23, revised 1996). The study was approved by the subcommittee on research animal care at our institution.
Forty-eight SD rats were divided randomly into six groups: the sham operation group (control group) and the ischemia/reperfusion injuryI-V group (IRI I-Vgroup). The trial rats were starved for 12 h but were allowed to drink water. A model of the partial warm hepatic ischemia/reperfusion injury was established at room temperature by the Nauta et al method[12]. In the experiment, the rats were anesthetized with aether. The liver was exposed by a midline incision before operation. The left lateral and median hepatic lobes were occluded with a microvascular clamp for 45 min. After 45 min of ischemia, reperfusion was induced by removing the vascular clamp. 0 h, 1 h, 6 h, 12 h and 24 h after the reperfusion, the rats were killed to isolate the Kupffer cells at the same time. The sham control rats underwent the same treatment, but without vascular occlusion.
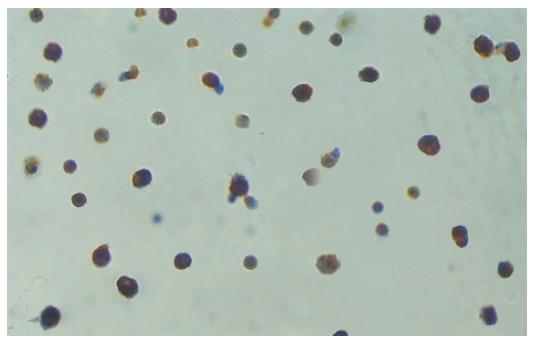
With the reference to the collagenase perfusion technique described by Knittel et al[13], the Kupffer cells were isolated by an improved method. In the experiment, the liver was perfused for 30 min with D-Hank’s containing 0.01% EDTA and removed to be smashed by scissors. The resulted stuff was digested for 45 min in a solution of 0.5 g/L type IV collagenase and was filtered through 200-mesh strainer. The filtrate was washed thoroughly and suspended in 3mL PBS, layered on 3 mL 25% percoll solution and 3 mL 50% percoll solution, centrifuged at 2500 r/min for 25 min to separate the cells. The cells between the layers of 25% percoll solution and 50% percoll solution were carefully extracted. The freshly isolated cells were suspended in DMEM medium supplemented with 25 mM HEPES, 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and L-glutamine (2 mmol/L). Cell suspensions (4 mL, 8 × 106 cells) were plated on 60-mm culture dishes and maintained in an incubator at 37°C in a humidified atmosphere of 90% air-100 mL/L CO2 for 1 h. The supernatants were discarded and the adherent cells were incubated for 4 h. The cellular components of adherent cells were examined by Lysozyme immunohistochemical staining to determine the percentage of Kupffer cells. The percentage of Kupffer cells in the adherent cells was over 90% (Figure 1). The cells were counted after the trypan blue exclusion. The viability of the cells was over 90% in the adherent cells. The Kupffer cells and supernatants were collected in all the groups and stored in refrigeration at -70°C for subsequent analysis.
TNF-α and IL-1β in the supernatants of primary cultures of rat Kupffer cells were measured by TNF-α and IL-1β ELISA kits. The concentrations of TNF-α and IL-1 were estimated based on the absorbance read by an ELISA reader (EL800) at 450 nm.
Immunocytochemiscal staining of Kupffer cells was performed with the strept-avidin-biotin-peroxidase complex (SABC) method.[14] Kupffer cells adhered to glass slides were fixed with aether and ethanol. They were soaked in 30% hydrogen and pure methanol (1:50) for 30 min at room temperature. After incubation in normal goat serum blocking solution, the glass slides were incubated in rabbit anti-rat MIP-1α polyclonal antibody (1:200) at 4°C overnight. PBS takes the place of MIP-1α antibody as the negative staining. Then the glass slides were incubated in biotined goat anti-rabbit IgG for 20 min at 20°C. Finally, the values of the mean optical density were measured under the same magnification using an image analysis system (original magnification × 400).
Total RNA was isolated from rat Kupffer cells using Trizol reagent. One microgram of RNA was reverse-transcribed to complement DNA using RNA PCR Kit according to the manufacturer’s instructions. The PCR primers were synthesized on the basis of Gen-Bank data. The primers were chemically synthesized using DNA synthesizer (Tiangen Biotech Co., Ltd, Beijing China). Their sequences were 5’-TTTGAGACCAGCAGCCTTTG-3’, 5’-GAAGAGTCCCTGGATGTGGC-3’ for MIP-1α and 5’- CATAGACAAGATGGTGAAGG -3’, 5’- TCCACAGTCTTCTGAGTGGC -3’ for GAPDH. All the PCR reactions had an initial denaturation step at 94°C for 3 min, and a final extension at 72°C for 5 min using PTC-100 (MJ Research, Inc. USA.). The PCR amplification cycling conditions were 94°C 30 s, 52°C 30 s, 72°C 30 s, 40 cycles for MIP-1α; 94°C 30 s, 53°C 30 s, 72°C 30 s, 35 cycles for GAPDH. Following RT-PCR, 5 μL samples of amplified products were resolved by electrophoresis in 1.5% agarose gel, and stained with ethidium bromide. The intensity of each PCR product was semiquantitatively evaluated using the labworks analysis software (UVP, Inc, Upland, CA, USA.).
Results were expressed as mean ± SE. Statistical calculations were made using the SPSS10.0 software package. One-factor analysis of variance was applied to determine whether the differences among the three groups were statistically significant. In all the cases, P values lower than 0.05 were considered to be statistically significant.
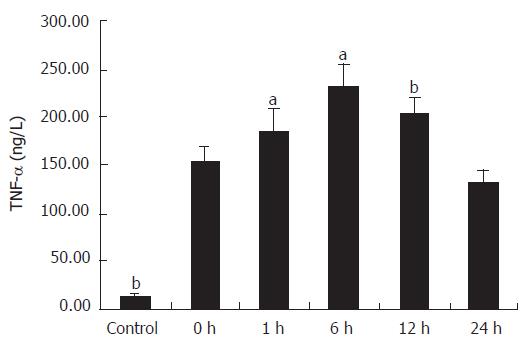
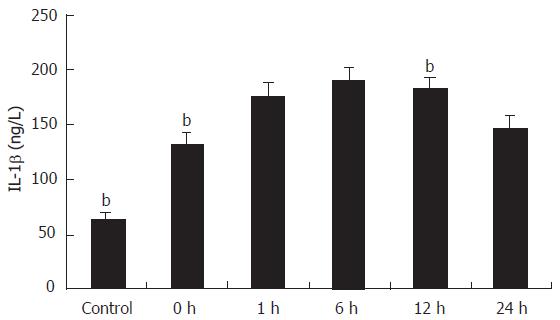
The cytokines released from the Kupffer cell were studied by ELISA. As shown in Figure 2, the concentration of TNF-α in the supernatant of primary cultures of rat Kupffer cells in the control group was 13.89 ± 2.04 ng/L and it increased at least ten times in IRI group I (P < 0.01). Following reperfusion injury stimulation, the TNF-α level was increased with time, and reached the maximum (230.6 ± 26.3 ng/L) 6 h after the stimulation (P < 0.01). Even 24 h after the reperfusion, the concentration of TNF-α (133.68 ± 12.15 ng/L) was still significantly high compared with that in the control group (P < 0.01). A comparison of IL-1β production in supernatant of Kupffer cells is shown in Figure 3. The concentration of IL-1β in the control group was 64.65 ± 4.63 ng/L and significantly increased with time, reaching its maximum (189.8 ± 13.13 ng/L) 6 h after the reperfusion (P < 0.01), and slightly declined (146.30 ± 11.90 ng/L) 24 h after the following reperfusion.
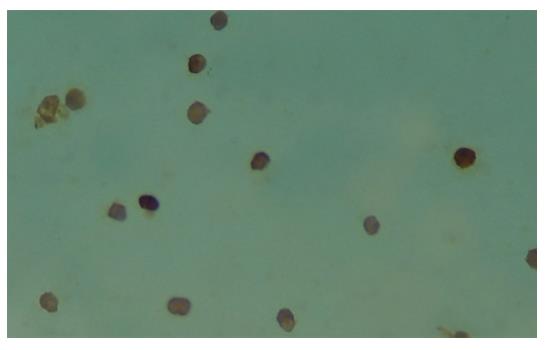
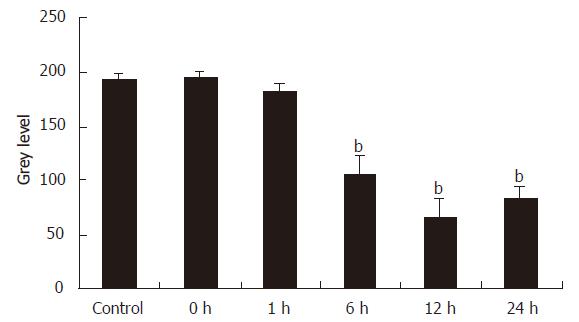
Figure 4 shows the immunohistochemical staining of the Kupffer cells that were isolated 12 h after the reperfusion of rat liver. The cytoplasms of these Kupffer cells were stained brown. On the contrary, in the control group, IRI group I and II, the Kupffer cells were not or weakly brown stained. We used an image analysis system to measure the values of the mean optical density of the Kupffer cells in all the groups and found the levels of MIP-1α protein in Kupffer cells had a significant increase at 6 h, 12 h and 24 h intervals (P < 0.01), which was contrary to the control group (Figure 5).
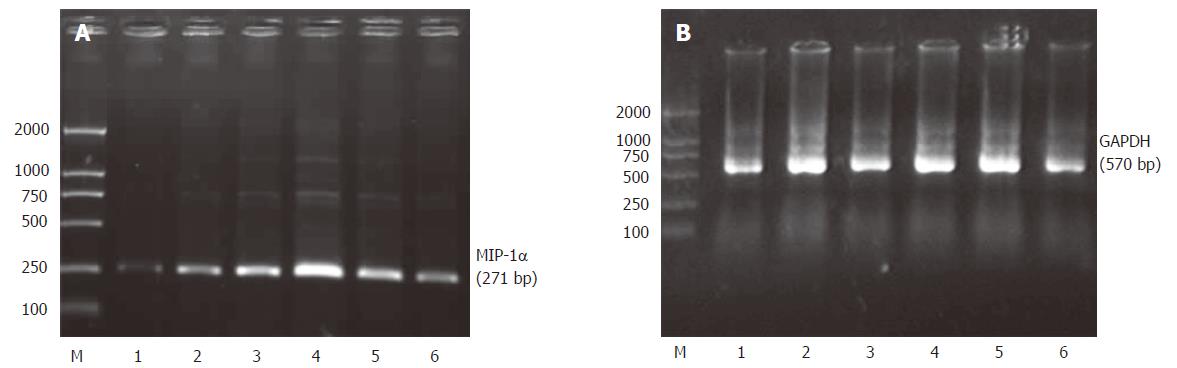
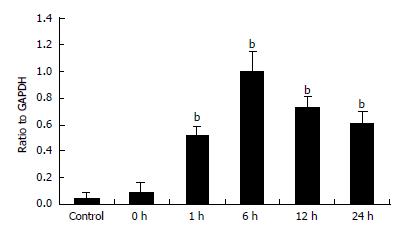
PCR products were electrophoresed on agarosegels and photographed (Figure 6). The track numbered 1 is for the control group and the tracks numbered 2-6 are for the ischemia/reperfusion injury I-V group. The track marked M is for DNA maker. Quantitative data of MIP-1α mRNA levels in Kupffer cells were represented by the ratio of relative absorbance and expressed as mean ± SD (Figure 7). It showed that the Kupffer cells in the control group had low but detectable levels of MIP-1α mRNA. There was no statistical significance between the control group and the ischemia/reperfusion injury I group (P > 0.05). The MIP-1α mRNA level in Kupffer cells significantly increased with time, reaching its maximum 6 h after the reperfusion injury (P < 0.01), and slightly declined at 24 h interval but was still much higher than that in the control group (P < 0.01).
Hepatic ischemia/reperfusion injury is one of the major complications of liver resection surgery, transplantation, and hypovolemic shock[1,15]. The detailed biochemical mechanisms of liver injury caused by ischemia/reperfusion are complex and not well known till now. Several animal modles were used to establish the pathological process of hepatic ischemia/reperfusion injury, such as the liver transplantation model, the partial warm or cold ischemia/reperfusion injury model and the total hepatic ischemia/reperfusion injury model[16-18]. In this study, the model of rat liver ischemia-reperfusion injury is established by the clamping and unclamping of the vessels to the left lateral and median hepatic lobes, which account for 70% of the rat’s liver mass. This hepatic insult is similar to the clinical situation where the whole liver is rendered ischemic during total vascular exclusion (which is not tolerated in the rat) for liver resections. We determine the outcomes 0 h, 1 h, 6 h, 12 h and 24 h respectively after the onset of reperfusion, when a number of mechanisms began to function, such as the increase in the cytokine production, the enhanced oxidative metabolism, and the increase in phagocytotic activity, which contribute to the hepatic injury[1,2]. Therefore, the findings of the present research reveal the pathological process of both ‘early and late’ phase ischemia/reperfusion injury.
Kupffer cells play an important part in mediating ischemia and reperfusion injury. When activated during the ischemia and subsequent reperfusion, they generate excessive inflammatory cytokines and oxygen-derived free radicals, which play a particularly important role in the pathogenesis of hepatic ischemia and reperfusion injury[19]. In this study, low levels of TNF-α and IL-1β were detected in the supernatant of primary cultures of rat Kupffer cells in the control group. At the early phase of the ischemia/reperfusion injury, they significantly increased and nearly reached its maximum. Twenty-four h after the reperfusion, these inflammatory cytokines were still significantly high compared with that in the control group. The mechanism of induction of TNF-α and IL-1β bioactivity after hepatic ischemia/reperfusion is complex. As a survival factor in hepatocytes under certain physiological conditions, for example, liver regeneration, Kupffer cells secrete low levels of TNF-α and IL-1β. During the very early phase of the hepatic ischemia/reperfusion injury, the activated Kupffer cells produce high concentrations of TNF-α and IL-1β that represents the results obtained 0 h and 1 h after the reperfusion. The secretions of these toxic inflammatory cytokines precedes the activation of adhesion factors, chemotactic agents, and the sequestration of neutrophils in the liver and appear to be a key mediator of the inflammatory response to promote the release of other cytokines.
Kupffer cells are both a target and a source of chemokines. Therefore, we also examine the expression of MIP-1α, an important member of CC chemokine subfamily, in Kupffer cells in an ischemia/reperfusion injury. The MIP-1α proteins are not detected by immunocytochemiscal staining in the cells of the control group and IRI group I and II. Likewise, the synthesis of MIP-1α mRNA in the cells of the control group and IRI group I are not active. After the reperfusion injury, the expression of MIP-1α protein and mRNA significantly increase. This may indicate that the genes MIP-1α are inactive in normal Kupffer cells. Some inflammatory factors stimulate MIP-1α gene in Kupffer cells during the reperfusion injury. These findings are in agreement with those in the research by Bukara M et al[20]. The role of MIP-1α in the pathological process of hepatic ischemia/reperfusion injury is not well known. However, previous studies have proved that MIP-1α can induce mononuclear macrophage to secrete TNF-α and IL-1β[11]. Therefore, we suppose the active behavior of the MIP-1α gene in the Kupffer cell following liver ischemia/reperfusion injury is assumed to be one of the major causes for the hepatic ischemia/reperfusion injury.
In conclusion, our study focuses on the immediate, innate immune response of the Kupffer cells and demonstrates the prominent expression of MIP-1α in the Kupffer cells in the hepatic ischemia/reperfusion injury. These theories may provide a better understanding of the mechanisms of hepatic ischemia/reperfusion injury and will be useful for the therapies of hepatic ischemia/reperfusion injury in the fields of hepatic surgery, preservation, and rejection.
The authors thank Dr Jiang Han, Pathophysiology Department, Xi’an Jiaotong University, for his help in technical assistance of Immunohistochemical staining. The excellent technical assistance of Dr. Zuo-Ren Wang is acknowledged.
S- Editor Wang J L- Editor Ma JY E- Editor Bai SH
| 1. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 358] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Strieter RM, Standiford TJ, Huffnagle GB, Colletti LM, Lukacs NW, Kunkel SL. "The good, the bad, and the ugly." The role of chemokines in models of human disease. J Immunol. 1996;156:3583-3586. [PubMed] |
| 4. | Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1546] [Cited by in RCA: 1489] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 5. | Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 308] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Taub DD. Chemokine-leukocyte interactions. The voodoo that they do so well. Cytokine Growth Factor Rev. 1996;7:355-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2697] [Cited by in RCA: 2686] [Article Influence: 95.9] [Reference Citation Analysis (4)] |
| 8. | Matsukawa A, Hogaboam CM, Lukacs NW, Kunkel SL. Chemokines and innate immunity. Rev Immunogenet. 2000;2:339-358. [PubMed] |
| 9. | Chaisavaneeyakorn S, Moore JM, Mirel L, Othoro C, Otieno J, Chaiyaroj SC, Shi YP, Nahlen BL, Lal AA, Udhayakumar V. Levels of macrophage inflammatory protein 1 alpha (MIP-1 alpha) and MIP-1 beta in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2003;10:631-636. [PubMed] |
| 10. | Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1803] [Cited by in RCA: 1796] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 11. | Kaplan AP, Kuna P, Reddigari SR. Chemokines and the allergic response. Exp Dermatol. 1995;4:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Nauta RJ, Tsimoyiannis E, Uribe M, Walsh DB, Miller D, Butterfield A. Oxygen-derived free radicals in hepatic ischemia and reperfusion injury in the rat. Surg Gynecol Obstet. 1990;171:120-125. [PubMed] |
| 13. | Knittel T, Fellmer P, Ramadori G. Gene expression and regulation of plasminogen activator inhibitor type I in hepatic stellate cells of rat liver. Gastroenterology. 1996;111:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Meng X, Zhang J. Study on expression and distribution of macrophage inflammatory protein-1α in atherosclerotic tunica intima of artery from type 2 diabetics by using inmunohistochemistry. China journal of modern medicine. 2005;15:20. |
| 15. | Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 17. | Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 174] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Kojima Y, Suzuki S, Tsuchiya Y, Konno H, Baba S, Nakamura S. Regulation of pro-inflammatory and anti-inflammatory cytokine responses by Kupffer cells in endotoxin-enhanced reperfusion injury after total hepatic ischemia. Transpl Int. 2003;16:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Zhang JX, Wu HS, Wang H, Zhang JH, Wang Y, Zheng QC. Protection against hepatic ischemia/reperfusion injury via downregulation of toll-like receptor 2 expression by inhibition of Kupffer cell function. World J Gastroenterol. 2005;11:4423-4426. [PubMed] |
| 20. | Bukara M, Bautista AP. Acute alcohol intoxication and gadolinium chloride attenuate endotoxin-induced release of CC chemokines in the rat. Alcohol. 2000;20:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |