Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3848
Revised: January 15, 2006
Accepted: January 19, 2006
Published online: June 28, 2006
AIM: To explore the possible mechanisms of curcumin in rat colitis induced by trinitrobenzene sulfonic (TNBS) acid.
METHODS: Rats with TNBS acid-induced colitis were treated with curcumin (30 mg/kg or 60 mg/kg per day ip). Changes of body weight and histological scores as well as survival rate were evaluated. Leukocyte infiltration was detected by myeloperoxidase (MPO) activity assay. The expression of cyclooxygenase-2 (COX-2) was detected by RT-PCR and Western blot. Inflammation cytokines were determined by RT-PCR. Local concentration of prostaglandin E2 (PGE2) in colon mucosa was determined by ELISA.
RESULTS: Curcumin improved survival rate and histological image, decreased the macroscopic scores and MPO activity. Also curcumin reduced the expression of COX-2 and inflammation cytokines. In addition, treatment with curcumin increased the PGE2 level.
CONCLUSION: Curcumin has therapeutic effects on TNBS acid-induced colitis, the mechanisms seem to be related to COX-2 inhibition and PGE2 improvement.
- Citation: Jiang H, Deng CS, Zhang M, Xia J. Curcumin-attenuated trinitrobenzene sulphonic acid induces chronic colitis by inhibiting expression of cyclooxygenase-2. World J Gastroenterol 2006; 12(24): 3848-3853
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3848
Inflammatory bowel disease (IBD) is characterized by chronic recurrent ulceration of the bowel. Frontline drugs that are currently used for the treatment of IBD include derivatives of 5-aminosalicvlic acid (5-ASA), glucocorticoid and immunosuppressives. Novel agents such as monoclonal antibodies against tumor necrosis factor alpha (TNF-α) are now commercially available[1,2]. However, these agents have varying efficacy and are expensive. Consequently, there is a need for alternative agents that may be equally or more effective and cheaper. Curcumin is the prominent yellow pigment in turmeric, a widely used spice and food colouring agent with anti-inflammatory and anti-cancer properties[3]. Mechanisms by which curcumin exerts its pharmacological effects are thought to involve antioxidation[4], inhibition of kinases, interference with the activity of transcription factors such as NF-κB and AP-1[5]. Cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) are inhibited by curcumin through NF-κB dependent or independent pathway[6,7].
In IBD, increased COX-2 mRNA and protein have been found in inflamed areas, and most of the PGs are produced by the COX-2 pathway. Hence, inhibition of excessive COX-2 activity and PGE2 production may result in suppression of the inflammatory response. Preventive and therapeutic mechanisms of curcumin are to inhibit the activation of NF-κB and reduce the activity of p38 MAPK[8-11]. But whether treatment of colitis with curcumin reduces COX-2 and PGE2 remains unknown. In the present study, we investigated the effect of curcumin on body weight changes, the expression of COX-2, the local concentration of PGE2 in colon mucosa and the changes of inflammatory genes in rats with colitis induced by trinitrobenzene sulphonic (TNBS) acid.
Specific pathogen-free Sprague-Dawley rats weighing 200-220 g were obtained from Laboratory Animal Center of Wuhan University (Wuhan, China) and kept at constant temperature (23 ± 2°C) and humidity (50%-70%) in a 12 h light and dark cycle. The rats were allowed to adapt to our laboratory environment for one week before experiment with free access to standard rodent chow and tap water.
Colitis was induced by TNBS acid using the modified method described by Morris et al[12]. Briefly, rats were fasted the day before colitis induction. Each rat was anesthetized with ether, and 100 mg/kg of TNBS acid (Sigma, St. Louis, MO, 100 g/L TNBS acid in 500 mL/L ethanol, total volume 1.0 mL) was then instilled via a rubber catheter inserted 8 cm into the colon via the anus. The rubber catheter was modified with numerous holes in the last 4 cm of its length. The instillation procedure required only a few seconds and the rats were maintained in a vertical position for 3 min to prevent solution leakage. Control mice received 500 mL/L ethanol of the same volume using the same technique. All the rats were checked daily for behavior and body mass. The experiments were approved by the Institution’s Animal Care and Use Committee.
To investigate the therapeutic and dosage effects of curcumin(≥ 95%, Sigma), rats were randomly divided into four groups: control group(n = 15, receiving ethanol only and no treatment), TNBS acid group(n = 15, receiving TNBS acid and no treatment), curL group(n = 15, receiving 30 mg/kg curcumin per day ip) and curH group(n = 15, receiving curcumin 60 mg/kg per day ip). On d 1, the rats were fasted, colitis was induced and treatment was begun on d 2. Con and TNBS acid groups received the same volume of 5% ethanol (vehicle of curcumin) everyday until the end of the experiment. Treated groups received curcumin just after colitis was induced for two weeks until the rats were sacrificed.
After rapid removal of the colon, specimens were flushed with ice cold PBS, cut open and photographed. The photographs of the colonic specimens were then scored by a blinded observer unaware of the treatment. Scores were assessed by using the following damage scoring system[12]: 0: no damage; 1: localized hyperaemia without ulcers; 2: linear ulcers with no significant inflammation; 3: linear ulcer with inflammation at one site; 4: two or more sites of ulceration and/or inflammation; 5: two or more major sites of inflammation and ulceration or one major site of inflammation and ulceration extending more than 1 cm along the colon. For histological analysis, tissues were fixed in 100 g/L paraformaldehyde in phosphate-buffered saline, and paraffin-embedded tissue sections were stained with hematoxylin and eosin (HE) using the standard techniques.
Myeloperoxidase (MPO) activity was assessed as a marker of neutrophil infiltration. Rat colon samples were snap-frozen in liquid nitrogen and stored at -80°C before MPO activity assessment. The tissue was thawed, weighed and homogenized in PBS, the homogenate was centrifuged and the pellet was again homogenized in PBS containing 5 g/L hexadecyl-trimethylammonium bromide (HETAB) and 10 mmol/L EDTA. This homogenate was subjected to three cycles of freezing/thawing and brief sonication. A sample of homogenates was added to reaction volume (containing 80 mmol/L PBS, pH 5.4, 5 g/L HETAB and 1.6 mmol/L 3, 3’, 5, 5’-tetramethylbenzidine). The mixture was incubated at 37°C for 5 min and the reaction was started by the addition of H2O2. The complete reaction mixture was incubated for exactly 3 min at 37°C and terminated by the sequential addition of catalase and sodium acetate. The changes in absorbance at 655 nm were measured with a spectrophotometer.
For RT-PCR, rat colon samples were snap-frozen in liquid nitrogen and stored at -80°C before ribonucleic acid (RNA) preparation. Total RNA was isolated using the TRIzol method (Life Technologies, Canada). Concentration of the RNA was detected from A260, and the integrity of the RNA was verified by electrophoresis on formaldehyde gels. Total RNA was reverse-transcribed into complementary deoxyribonucleic acids (cDNAs) using a first strand cDNA synthesis kit (Fermentas, Life Sciences).The resultant cDNAs were subjected to PCR for measurement of messenger RNAs (mRNAs). The PCR products were subjected to agarose gel electrophoresis and the abundance of each mRNA was normalized to that of GAPDH. The sequences of all primers used in this project are as flowing:
GAPDH: sense: 5’ATGGGTGTGAACCACGAGAAA-3’,
anti-sense: 5’GGATACATTGGGGGTAGG-AA-3’(330 bp);
COX-2: sense: 5’-TACAAGCAGTGGCAAAGGC-3’,
anti-sense: 5’-CAGTATTGAGGAGAACAGATGGG-3’(304 bp);
TNF-α: sense: 5’-TACTGAACTTCGGGGTGATTGGTCC-3’,
anti-sense: 5’-CAGCCTTGTCCCTTGAAGAGAACC-3’(295 bp);
IFN- sense:5’AGCCTAGAAAGTCTGAAGAAC-3’,
anti-sense:5’ACCGACTCCTTTTCCGCTTC-CT-3’(387 bp)
iNOS: sense:5’-TGAAGCACATGCAGAAATGAGTACCG-3’,
anti-sense: 5’-CCGTCAGAGGTAACTGTTTACACG-3’ (464 bp)
Western blot analysis was performed by standard methods. Briefly, frozen tissue samples were homogenized in Tris-HCl buffer containing a cocktail of protease inhibitors and insoluble materials removed by centrifugation at 4°C. The solubilized lysates were resolved by sodium dodecyl sulfate (SDS)-PAGE electrophoresis under reducing conditions at a concentration of 50 μg protein of each sample per lane. Nitrocellulose membranes were incubated overnight with rabbit anti-serum directed against COX-2 (Santa Cruz Biotechnology). Immunodetection with secondary peroxidase-conjugated antibody and chemiluminescence was performed according to the manufacturer’s protocol (Santa Cruz Biotechnology). Density of the products was quantified.
Colonic mucosal samples kept at -80°C were weighed and homogenized on ice in 1 mL Tris-HCl buffer containing 5.6 mmol/L indomethacin (pH 7.4), the homogenization was vortexed thoroughly for at least 2 min. After centrifugation, the concentration of PGE2 in supernatants was immediately determined with competitive ELISA kits (R&D), according to the manufacturer’s instructions. PGE2 levels were normalized to microgram of protein. The detection limit of PGE2 was 39 ng/L.
Data were expressed as mean ± SE. The statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s test. P < 0.05 was considered statistically significant. In the experiment involving histology, the figures shown are representative of at least three experiments performed on different days.
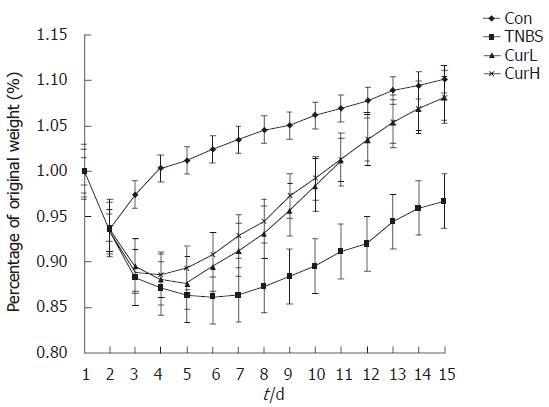
Administration of TNBS acid caused a dramatically decrease in body mass of all groups. Most of them decreased more than 30 g after 2 d (data not shown). Con group showed transient and slight loss of body weight (less than 10 g, data not shown) and recovered quickly. From d 3 it began to show significant difference from TNBS acid group (P < 0.05, Figure 1). At the end of the experiment, except for TNBS acid group, body weight of the other groups all reached the initial level. No difference was observed among control, curL and curH groups at the end of the experiment.
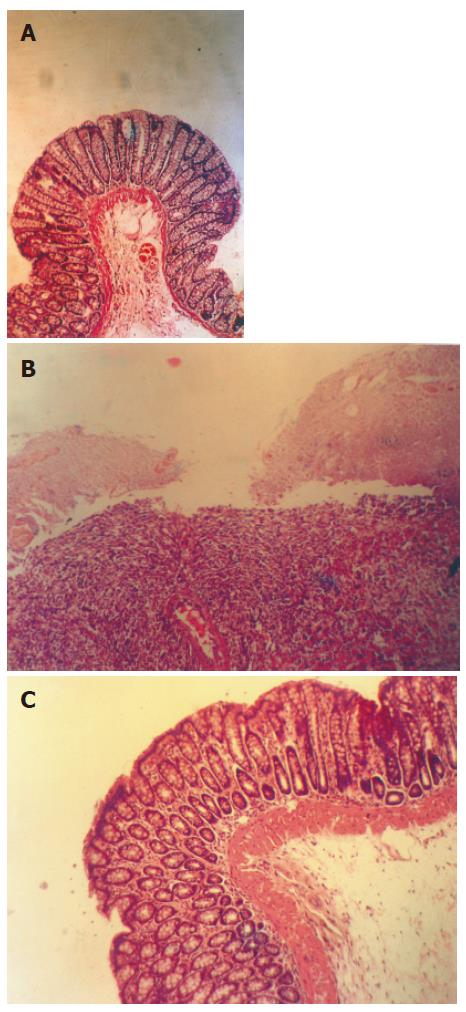
TNBS acid-induced colitis was characterized by thick and stiff colonic wall due to edema, chemical erosion or fibriform-proliferation. Ulcers were scattered along the colon or linked with each other, bleeding or redness was observed in whole or partial colon. Necrosis of epithelium, distortion of crypts, destruction of glands and infiltration of inflammatory cells were observed. The macroscopic scores in curL and curH groups were significantly lower than those in TNBS acid group (P < 0.01 Figure 2). Treatment with curcumin decreased edema. Ulcers in curcumin-treated groups were much smaller and superficial, most of the ulcers were healed and granulation or fibroplasias could be seen. Histological analysis showed that curcumin treatment decreased necrosis of epithelium and the infiltration of inflammatory cells and granulation can be seen.
Myeloperoxidase activity was significantly (P < 0.01) increased in TNBS acid-treated animals compared with control group (101 020 ± 17 170 nkat/g and 249 382 ±43 342 nkat/g tissue protein, respectively, Table 1 ), which was consistent with the histological findings. Treatment with curcumin (30 mg/kg or 60 mg/kg per day) significantly reduced the degree of polymorphonuclear and neutrophil infiltration in rats with TNBS acid-induced colitis.
| Tissue PGE2 (ng/g) | Protein MPO (knat/g) | TNF-α | mRNA IFN-γ | iNOS | |
| Con 15 | 176.3 ± 29.2c | 101 020 ± 17 170d | 0.0795 ± 0.0096d | 0.0284 ± 0.0150d | 0.8304 ± 0.1180d |
| TNBS 8 | 78.5 ± 13.2a | 249 382 ± 43 342b | 0.8861 ± 0.0533b | 1.0212 ± 0.0687b | 6.5690 ± 0.9450b |
| CurL 12 | 190.2 ± 17.0c | 121 691 ± 23 171ad | 0.1967 ± 0.0365bd | 0.0565 ± 0.0177ad | 1.8638 ± 0.3347bd |
| CurH 13 | 215.5 ± 20.4ac | 122 191 ± 21 171ad | 0.1727 ± 0.0348bd | 0.0518 ± 0.0057d | 1.7250 ± 0.3026bd |
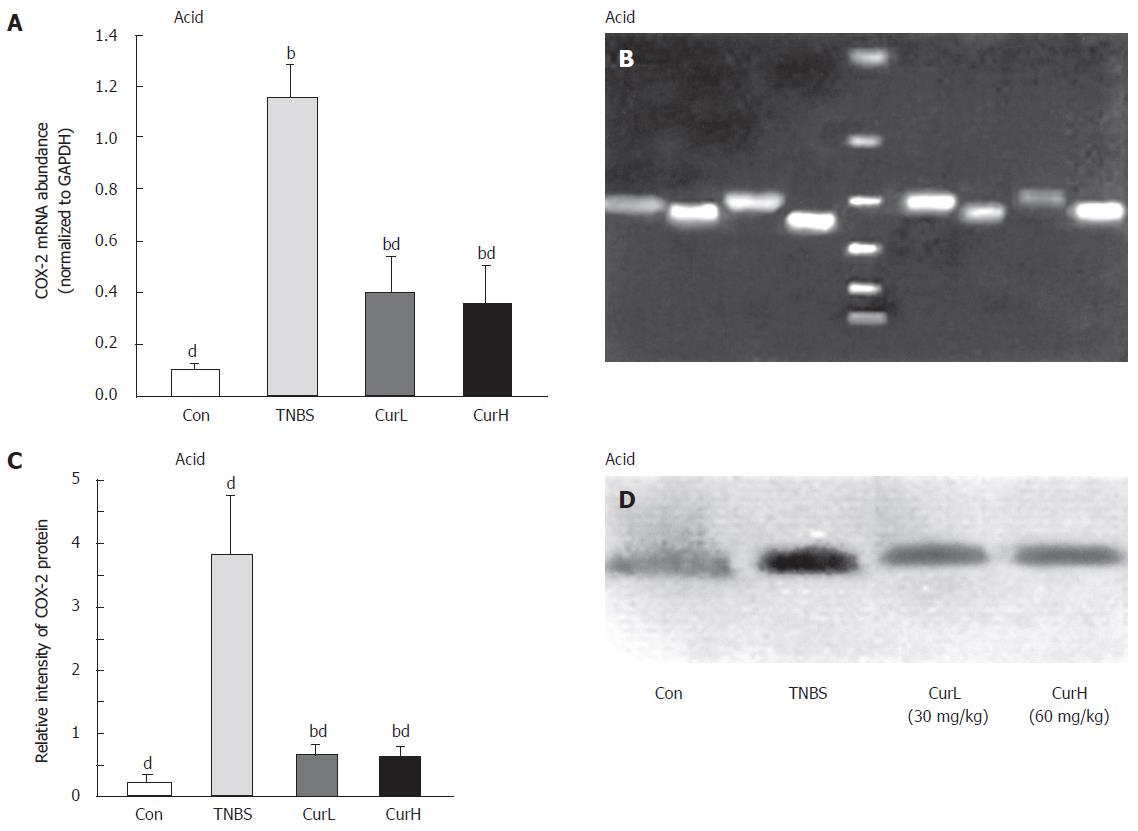
Weak expression of COX-2 mRNA could be detected in normal colon (Figure 3). After colitis was induced by TNBS acid, its expression increased by more than 10-fold. Treatment with curcumin decreased its expression by 70%, but was still higher than normal (P < 0.05). Western blot analysis showed the similar changes of protein in colon mucosa (Figure 3). After colitis was induced, PGE2 decreased and its expression was increased after curcumin treatment.
Abundance of IFN-γ, TNF-α and iNOS mRNA in TNBS acid group was significantly elevated compared with control group. Curcumin treatment suppressed the increased mRNA efficiently, but its level was still higher than normal (Table 1).
Treatment with curcumin (30 mg/kg or 60 mg/kg per day) reduced the severity and extension of damage induced by TNBS acid, decreased the extent of colitis and increased the survival rate and the incidence of adhesions. The rats treated with curcumin had improved histological image and less neutrophil infiltration in colon mucosa. The expression of COX-2 increased in acute and chronic colitis, suggesting that over-expression of COX-2 may result in neoplasia[13-17]. Curcumin exerts its antitumor and anti-inflammation effects by down-regulating COX-2 directly or indirectly[18]. Some reports indicate that selective COX-2 inhibitors exacerbate colitis[19-21], but others indicate that they have therapeutic effects on colitis[22-26]. In our experiment, the expression of COX-2 increased in colon following the induction of colitis, suggesting that high expression of COX-2 in the colon is associated with high MPO activity and cytokine production. Curcumin decreased the level of COX-2, indicating that decreasing the expression of COX-2 may benefit to colitis.
PGE2, an inflammatory mediator, participates in epithelium repairing and exerts anti-inflammation effects including suppression of neutrophil function or prevention of mast cell degranulation. Recently, PGE2 has been implicated in the inhibition of the production of IFN-γ, IL-2 and IL-12[27]. It was reported that in early or acute inflammation phase the expression of PGE2 is usually up-regulated, resulting in inflammatory manifestations, such as redness, edema, pyrexia and ache[15,28]. Treatment with curcumin increased the expression of PGE2 though its limiting enzyme COX-2 decreased, indicating that curcumin can change the expression spectrum of PGs in colitis.
TNBS acid-induced chronic intestinal inflammation mimics human Crohn’s disease, showing high IFN-γ, TNF-α and iNOS, but low levels of IL-4 and IL-5[19,29]. Treatment with curcumin decreased IFN-γ, TNF-α and iNOS distinctly in our study, which is consistent with the reports of Ukil et al[11] and Sugimoto et al[9]. Since PGE2 is capable of inhibiting Th1 cytokines, the decreased IFN-γ, TNF-α and iNOS in this study maybe due to PGE2. Although curcumin decreased the expression of inflammatory response genes, but not decrease it to the normal level, suggesting that curcumin is not able to completely eliminate colitis.
Curcumin (30 mg/kg or 60 mg/kg per day) did not show any difference in the treatment of colitis induced by TNBS acid. Curcumin is also a chemopreventive drug for many tumors[18]. IBD patients are at risk of developing dysplasia and neoplasia. Phase I clinical trial of curcumin indicated that 8 g/d for 3 mo does not show any toxicity[30], suggesting that long-term administration of curcumin in IBD patients is not only safe but also improves colitis and prevents development of colorectal cancer.
In conclusion, curcumin attenuates TNBS acid-induced chronic colitis by decreasing the expression of COX-2 and increasing PGE2. Curcumin can down regulate the expression of inflammatory genes in colitis induced by TNBS acid and is a safe and therapeutic agent for IBD patients
S- Editor Pan BR L- Editor Wang XL E- Editor Bai SH
| 1. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. [PubMed] |
| 2. | Tsujikawa T, Nezu R, Andoh A, Saotome T, Araki Y, Ishizuka Y, Sasaki M, Koyama S, Fujiyama Y. Inflixmab as a possible treatment for the hemorrhagic type of Crohn's disease. J Gastroenterol. 2004;39:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1-7. [PubMed] [DOI] [Full Text] |
| 4. | Barclay LR, Vinqvist MR, Mukai K, Goto H, Hashimoto Y, Tokunaga A, Uno H. On the antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Org Lett. 2000;2:2841-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Huang TS, Lee SC, Lin JK. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1991;88:5292-5296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 275] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, Kim SS. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004;94:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813-819. [PubMed] |
| 8. | Salh B, Assi K, Templeman V, Parhar K, Owen D, Gómez-Muñoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235-G243. [PubMed] |
| 9. | Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005;11:1747-1752. [PubMed] |
| 11. | Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 13. | Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 363] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Murakami A, Hayashi R, Tanaka T, Kwon KH, Ohigashi H, Safitri R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66:1253-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL-10(-/-) mice. Gastroenterology. 2000;118:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 18. | Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 572] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 19. | Colón AL, Menchén LA, Hurtado O, De Cristóbal J, Lizasoain I, Leza JC, Lorenzo P, Moro MA. Implication of TNF-alpha convertase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine. 2001;16:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Radaelli F, Feltri M, Meucci G, Spinzi G, Terruzzi V, Minoli G. Ischemic colitis associated with rofecoxib. Dig Liver Dis. 2005;37:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Singh VP, Patil CS, Jain NK, Kulkarni SK. Aggravation of inflammatory bowel disease by cyclooxygenase-2 inhibitors in rats. Pharmacology. 2004;72:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 22. | Biancone L, Tosti C, De Nigris F, Fantini M, Pallone F. Selective cyclooxygenase-2 inhibitors and relapse of inflammatory bowel disease. Gastroenterology. 2003;125:637-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Mahadevan U, Loftus EV, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002;97:910-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Kankuri E, Vaali K, Korpela R, Paakkari I, Vapaatalo H, Moilanen E. Effects of a COX-2 preferential agent nimesulide on TNBS-induced acute inflammation in the gut. Inflammation. 2001;25:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Karmeli F, Cohen P, Rachmilewitz D. Cyclo-oxygenase-2 inhibitors ameliorate the severity of experimental colitis in rats. Eur J Gastroenterol Hepatol. 2000;12:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Martín AR, Villegas I, Alarcón de la Lastra C. The COX-2 inhibitor, rofecoxib, ameliorates dextran sulphate sodium induced colitis in mice. Inflamm Res. 2005;54:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 875] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 28. | Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 976] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 29. | Egi H, Hayamizu K, Yoshimitsu M, Shimamoto F, Oishi K, Ohmori I, Okajima M, Asahara T. Regulation of T helper type-1 immunity in hapten-induced colitis by host pretreatment with granulocyte colony-stimulating factor. Cytokine. 2003;23:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895-2900. [PubMed] |