INTRODUCTION
Alpha-fetoprotein (AFP) is a major serum protein synthesized by fetal liver cells, yolk sac cells, and in trace amounts by the fetal gastrointestinal tract[1]. AFP appears to function as an osmotic and carrier protein in the fetus and to regulate the immune system by immunosuppressive functions, such as preventing immunological attacks to the embryo by the maternal immune system[1]. Reappearance of AFP in adult serum often signals pathological conditions, particularly the presence of hepatocellular carcinoma (HCC) and germ cell tumors containing yolk sac cell elements[2]. Now AFP has been successfully used as a diagnostic and prognostic tool for HCC. Although many investigations for the function of AFP had been carried out, the biological role of AFP is still a riddle so far.
Heat shock proteins (HSPs) are molecular chaperones which are emerging as biochemical regulators of cell growth, apoptosis, protein homeostasis and cellular targets of peptides[3-5]. Up regulated expression of HSPs during the growth of cancer cells has a close relationship with cell proliferation[6-8]. It is interesting to note that several studies have shown that AFP plays some roles during cell survival and proliferation of HCC cells[9-11]. Previous studies have confirmed overexpression of HSP70 in HCC[12]. So there may be a possible correlation between the expression of HSP70 and AFP during the growth and differentiation of HCC cells. In this study, by immunocytochemistry and immunoprecipitation, we observed that HSP70 chaperoned AFP in HCC cell cytoplasm. The interaction between HSP70 and AFP in human HCC cells will provide a new route for studying the pathogenesis and immunotherapy of HCC.
MATERIALS AND METHODS
Reagents
Rabbit anti-human HSP70 antibody, mouse anti-human AFP monoclonal antibody, TRITC labeled goat anti-rabbit antibody and FITC labeled goat anti-mouse antibody were purchased from Santa Cruz Company. EnVisionTM kits were purchased from Dako Biological Technology Company. ProteinA/G-agarose beads were purchased from Gene Company.
Cell line and cell culture
Human HCC cell line BEL-7402 was provided by the Institute of Oncology, Chinese Academy of Medical Sciences, Beijing, China. The derived cell lines were grown in RPMI 1 640 medium supplemented with 100 mL/L heat-inactivated fetal calf serum, 50 000 U/L penicillin and 0.05 g/L streptomycin. The cells were maintained at 37 °C in a humidified atmosphere containing 50 mL/L CO2. Viability of the cells used in these experiments was consistently more than 95% when evaluated by the trypan blue exclusion method. Cells at a density of 2.5105/mL were seeded into six-well plates for 24 h, washed with PBS, fixed by adding 2 mL of cold 500 mL/L methanol-acetone and stored at 4 °C for 20 min, then washed with PBS, and dried at room temperature.
Immunocytochemistry
All cells in six-well plates were hydrated with graded alcohol. Endogenous peroxidase was then blocked with 3 mL/L H2O2 diluted in methanol for 30 min at room temperature. Antigen retrieval was performed by treating the slides in citrate buffer in a microwave for 10 min. The cells were incubated in a moist chamber with HSP70 rabbit antibody (1:100) or AFP mouse monoclonal antibody (1:100) at 4 °C overnight, respectively. After a complete wash in PBS, the cells were treated with HRP labeled goat anti-rabbit and goat anti-mouse antibody (1:100) for 45 min at 37 °C. After a complete wash in PBS, all cells were developed in 0.5 g/L freshly prepared diaminobenzidine solution (DAB, Sigma Co.) for 8 min, and then counterstained with hematoxylin, dehydrated, air dried, and mounted. Anti-HSA was used to substitute for the primary antibody as a negative control.
Indirect double-immunofluorescence cytochemistry
All cells in six-well plates were hydrated with graded alcohol. The cells were blocked in 10 mL/L bovine serum albumin (BSA) for 30 min at room temperature, and then incubated with HSP70 rabbit antibody (1:100) or AFP mouse monoclonal antibody (1:100) at 4 °C overnight, respectively. After a complete wash in PBS, the cells were treated with TRITC labeled goat anti-rabbit antibody or FITC labeled goat anti-mouse antibody (1:20) for 40 min at room temperature. After extensive washing, the stained cells were observed under an immunofluorescence microscope. Anti-HSA was used to substitute for the primary antibody as a negative control.
Immunoprecipitation and Western blot
Cells at a density of 2.5105/mL were seeded into six-well plates for 24 h. Then cells were treated with 0.5 g/L trypsin and 0.2 g/L EDTA. After washing with cold PBS, the cells were centrifuged and harvested. They were then lysed with 500 μL of lysis buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mL/L Triton X-100, 10 g/L sodium deoxycholate) containing 1 µg/mL pepstatin and 1 mmol/L phenyl-methylsulfonyl fluoride (PMSF). Also, five units of apyrase (Sigma, Co.) were added to the lysate to deplete endogenous ATP. The cell lysates were sonicated and clarified by centrifugation. The supernatants were preabsorbed with 20 μL protein A/G-agarose beads at 4 °C for 4 h. After centrifugation, the supernatants were incubated with 100 μL HSP70 rabbit antibody (1:100) or 100 μL AFP mouse monoclonal antibody (1:100), respectively at 4 °C for 60 min. Then 50 μL protein A/G-agarose beads were added and incubated for 60 min at 4 °C. The immunoprecipitates were collected by centrifugation and washed five times with PBS. The precipitated protein complexes were released from the immunopellet with SDS-sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 25 g/L SDS, 50 mL/L β-mercaptoethanol, 100 mL/L glycerol) at 100 °C for 5 min. The immunop-recipitates were then analyzed by 90 g/L SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose (NC) membrane (BioRad) and detected by immunoblotting with anti-AFP monoclonal antibody or anti-HSP70 antibody (1:100) at 4 °C overnight, respectively. After a complete wash in PBS, the membranes were treated with HRP labeled goat anti-rabbit and goat anti-mouse antibody (1:100) for 45 min at 37 °C. After a complete wash in PBS, the membranes were developed in 0.5 g/L freshly prepared diaminobenzidine solution (DAB, Sigma Co.) for 8 min. Anti-HSA was used to substitute for the primary antibody as a negative control.
Statistical analysis
HSP70, AFP expression differences between HCC cell BEL-7402 and control group were analyzed statistically using χ2-test. P<0.05 was considered statistically significant.
RESULTS
Expression of HSP70 and AFP in HCC cell line BEL-7402
Positive expression of HSP70 and AFP showed brown staining in the nuclei or cytoplasm, more than 500 cells calculated in different microscopic fields of each well, and the percentage of positive cells were evaluated.
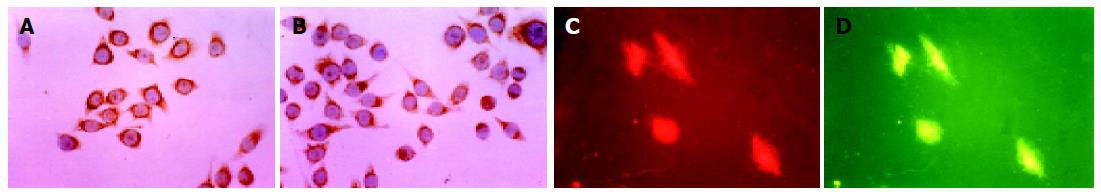
Immunocytochemical staining showed that the positive rate of HSP70 was 96.0% (480/500), and AFP was 100.0% (500/500), while control group was 5.0% (25/500). There was a significant difference between experimental group and control group (P<0.01). HSP70 and AFP were mainly stained in cell cytoplasm (Figure 1).
Figure 1 Expressions of HSP70 and AFP in HCC cell line BEL-7402 by immunocytochemistry and immunofluorescence cytochemistry, ×400.
A: HSP70 positive expression in cytoplasm; B: AFP immunostaining in cytoplasm; C: HSP70 immunostaining in cytoplasm; D: AFP green immunofluorescence in cytoplasm.
Interaction between HSP70 and AFP in HCC cell line BEL-7402
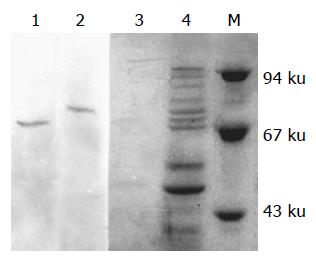
Immunoprecipitate and Western blot showed that there were two clear protein bands in the transferred NC membrane. Under-precipitated with anti-HSP70 antibody, there appeared a Mr 68 000 clear AFP band (lane 1) and under-precipitated with anti-AFP mAb, there existed a Mr 72 000 clear HSP70 band (lane 2), demonstrating that AFP existed in the immunoprecipitate of anti-HSP70 antibody and HSP70 existed in that of anti-AFP mAb. The results indicated that HSP70 interacted with AFP in HCC cell line BEL-7402 (Figure 2).
Figure 2 Analysis of immunoprecipitate of anti-HSP70 antibody and anti-AFP mAb by SDS-PAGE and Western blot (lane 1: precipitated with anti-HSP70 antibody; lane 2: precipitated with anti-AFP mAb; lane 3: precipitated with anti-HSA antibody; lane 4: cell lysates supernatants; M: molecular mass markers).
DISCUSSION
In this study we examined the expressions of HSP70 and AFP in HCC cell line BEL-7402 by immunocytochemistry, immunofluorescence cytochemistry and immunoprecipitate analysis. The results showed that almost all HCC cells detected expressed a high level of HSP70 and AFP, which had a significant difference compared with control group. HSP70 chaperoned AFP that mainly localized in cell cytoplasm.
AFP always accompanies the growth of liver cells, and it is possible that AFP may be related to the proliferation of tumor or fetal cells[8-10]. The mechanism for growth-promoting activity of AFP is still unclear. Escaping from the surveillance of immune system is the primary cause for malignant growth of HCC cells[12,13]. Several investigations have shown that AFP could be individually synergistic with other growth factors to promote the growth of many tumor cells[14,15]. AFP receptors have been found anchoring on the membrane of various tumor cells[16-17]. The receptor may mediate intercellular signal transduction which influences the expression of genes related to proliferation[16,17]. AFP can stimulate the expression of some oncogenes which controls cell cycle, and then enhance the proliferation of human HCC[19,20]. When BEL-7402 cell line were treated with AFP, oncogene protein, such as c-fos, c-jun, n-ras and mutative p53 and p21ras increased rapidly, which have an important function in modulating growth and differentiation of the cells.
Heat shock protein (HSP) is a group of highly conserved proteins synthesized after heat induction[3-5]. In normal cells, HSP70 is constitutively expressed at low levels but the expression was dramatically enhanced by stressful conditions[3]. HSPs are thought to act as molecular chaperone, helping in transporting, folding and processing of their target proteins. Although the cellular implication of the increased production of these proteins is unknown, it may be expected that each response would enhance the capacity of the pathway(s) in which these proteins function and perhaps protect the associated cellular compartments from damage via abnormal protein interactions. Enhanced expression of HSPs during the growth of cancer cells implies its close relationship with cell growth[6-8]. It is interesting to note that AFP plays some roles during tumor cell survival and proliferation[9-11]. These studies have suggested a possible correlation between the overexpression of HSP70 and AFP during the growth and differentiation of tumor cells. Continuous expression of HSP70 in tumor cells is required to serve as molecular chaperones in regulating and stabilizing tumor growth process. Some studies have suggested that high level expression of HSP70 could contribute to tumorigenecity of certain tumors[7,8], but its role in tumorigenecity is not clear. So, it is reasonable to propose that HSP70 and AFP up-expression in these tumor cells is closely related with the tumor cell survival and proliferation. Up-regulated expression of HSP70 in tumor cells may be required to serve as molecular chaperones in regulating and stabilizing oncofetal protein and mutant oncogene products during the tumor growth process[21,22]. Therefore, HSP70 could assist to stabilize AFP in cell cytoplasm, transport it to cell membrane and release its monomer to the serum, which indirectly prompts and protects AFP function. Conversely, AFP itself stimulates HCC cell proliferation through membrane receptor mediating cellular signal transduction pathways. This indicates that the proliferating HCC cells need much more HSP70 to maintain the formation of AFP activities. Our results showed that, in human HCC BEL-7402 cell line, there exists a high level of expression of HSP70 and AFP. It indicates that co-expression of HSP70 and AFP is likely to have some relationship with proliferation, development and poor prognosis of human HCC. The observed fact is that HSP70 chaperoned AFP may be useful to study the pathogenesis and immunity of human HCC.
Recent studies on the immunodominant epitopes of AFP have provided a solution to the obstacle of HCC immun-otherapy. AFP is produced at low serum levels after birth throughout life[1]. The majority of human HCC overexpress the oncofetal antigen AFP, Mr 64 000-72 000 glycoprotein[2]. Despite being exposed to high plasma levels of this oncofetal protein during embryonic development, the body has a low immunity to it[3-5]. Butterfield et al[23], recently found that four peptides of human AFP processed and presented in the context of HLA-A0201, could be recognized by the human T cell repertoire, and could be used to generate AFP-specific CTL in human T cell cultures. It was also found that murine immune system could generate T-cell responses to this oncofetal antigen[24]. Therefore, it may be a better target for immunotherapy. But AFP immunization alone still results in lower levels of specific response and poorly reproducible protective immunity[24,25].
How to enhance the host’s active immunity to AFP may be an interesting strategy for HCC therapy. In our previous study, tumor rejection assay demonstrated that recombinant DNA vaccine AFP/HSP70 elicited strong specific antitumor immunity against AFP-producing SP2/0 cells than AFP DNA vaccine. Results indicated that AFP immunogenicity can be improved greatly by HSP70 molecular and vaccination with DNA encoding HSP70 could increase both humoral and T-cell proliferation responses to AFP. We attributed the successful AFP specific T-cell responses in mice to the HSP70 molecule by mediating APCs for efficient uptake and process of AFP. Numerous investigations have been verified that HSP70 is a better molecular chaperone and adjuvant which can process and present weak tumor antigen to MHC-I of host APCs, eliciting specific T-cell response and CTL reaction[26,27]. HSP70-associated peptides can also anchor antigen on the cell membrane and directly present it to nature killer cells or γδ T cells as superantigen without being dependent on the stimulation of MHC-I molecules[28,29]. The observed fact is that HSP70 chaperoned AFP in HCC, may be useful to study tumor pathogenesis or design effective vaccine against liver cancers.