Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.4947
Revised: February 15, 2005
Accepted: February 18, 2005
Published online: August 28, 2005
AIM: In this study, a hepatocyte-specific targeting technology was developed by modifying cationic liposomes with soybean sterylglucoside (SG) and polyethylene glycol (PEG) (C/SG/PEG-liposomes).
METHODS: The liposomal transfection efficiencies in HepG2 2.2.15 cells were estimated with the use of fluorescein sodium (FS) as a model drug, by flow cytometry. The antisense activity of C/SG/PEG-liposomes entrapped antisense oligonucleotides (ODN) was determined as HBsAg and HBeAg in HepG2 2.2.15 cells by ELISA. The liposome uptake by liver and liver cells in mice was carried out after intravenous injection of 3H-labeled liposomes.
RESULTS: C/SG-liposomes entrapped FS were effectively transfected into HepG2 2.2.15 cells in vitro. C/SG/PEG-liposomes entrapped ODN, reduced the secretion of both HBsAg and HBeAg in HepG2 2.2.15 cells when compared to free ODN. After in vivo injection of 3H-labeled C/SG/PEG-liposomes, higher radiation accumulation was observed in the hepatocytes than non-parenchymal cells of the liver.
CONCLUSION: C/SG/PEG-liposomes mediated gene transfer to the liver is an effective gene-delivery method for hepatocytes-specific targeting, which appears to have a potential for gene therapy of HBV infections.
- Citation: Qi XR, Yan WW, Shi J. Hepatocytes targeting of cationic liposomes modified with soybean sterylglucoside and polyethylene glycol. World J Gastroenterol 2005; 11(32): 4947-4952
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/4947.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.4947
Cationic liposomes have been accepted as effective non-viral vectors for gene delivery with a lower immunogenicity than the viral ones. However, the lack of organ or cell specificity sometimes hampers their applications. In the case of cationic liposomes, the highest gene expression is observed in the lung after intravenous injection of their plasmid DNA complexes in most cases, because the lung capillaries are the first traps to be encountered[1,2]. Development of cell-specific targeting technology for cationic liposomes attracts great interest in gene therapy.
Since the liver is one of most important target organs in the body, and Kupffer cells in the liver are a part of the reticular endothelial system (RES), relatively high accumulation of administered liposomes is observed in the liver, mostly in non-parenchymal cells[3]. However, preferential incorporation of liposomes into liver hepatocytes is required for therapeutic situations. Thus, reducing the Kupffer cell uptake and enhancing hepatocyte uptake, are challenges of research in liposome-targeting.
For cell specific delivery, the receptor-mediated endocytosis systems endowed to various cell types would be useful and a number of gene delivery systems have been developed to introduce gene into specific cells with receptor-mediated endocytosis. Ligands currently being investigated include galactose[4,5], lactose[6], transferring[7], etc. Among these receptors, asialoglycoprotein receptor (ASGP-R) is the most promising for gene targeting, since it exhibits high affinity and a rapid internalization rate[8].
It was reported that liposomes with soybean sterylgl-ucoside (SG) gets accumulated in the liver, especially in hepatocytes[9,10]. Doxorubicin (DXR) entrapped in liposomes contained SG (SG-liposomes) showed a high therapeutic effect for its selective delivery of drugs to hepatocytes in animals with liver cancer[11]. SG-liposomes have glucose residue on the surface of liposomes[12], which is essential for selective accumulation in liver cells.
Hepatitis B is a disease of global importance with more than 300 million carriers of the hepatitis B virus (HBV) worldwide[13]. Unfortunately, treatment of chronic HBV infection is far from satisfactory. The most successful therapeutic agent so far available is interferon-alpha, which shows a 40% response rate for patients after completion of the therapy[14,15]. Since several viruses have become successful targets of the ODN approach, this strategy may be promising in targeting chronic HBV infection, and several studies have now shown that ODN are capable of suppressing HBV in vitro[16,17] and in vivo[18,19]. ODN are synthetic single chain DNA molecules that can inhibit gene expression within cells by their capability to bind a complementary mRNA sequence, and prevent translation of mRNA, thus providing potentially powerful therapeutic tools against viral diseases and cancer[20]. For an ODN delivery system towards HBV infection, the SG may be useful for targeting hepatocytes of the liver.
This study describes a specific targeting approach which results in increased hepatocytes uptake. A cationic liposome carrier modified with SG and encapsulated 15-mer ODN for the HBV therapy in vitro was constructed. The influence of SG on facilitating the uptake of liposomes by hepatocytes was investigated in vivo. The value of the surface modified cationic liposomes as a delivery vehicle, mainly for hepatocytes targeting antisense agent in vitro and in vivo was assessed.
ODN with phosphorothioate backbone encoding the cap site of SP II promoter transcribed mRNA (cap site/SP II) sequences were synthesized using standard phosphoramadite chemistry by Aoke (Beijing, China) and purified by SDS-PAGE. The complementary ODN sequences were: 5’ GAT GAC TGT CTC TTA 3’.
Male KM mice (18-23 g) were obtained from the Institute of Zoology, Chinese Academy of Sciences (Beijing, China). All animals received good care. A human hepatoblastoma cell line, HepG2 2.2.15 was provided by the Institute of Hepatology of the People’s Hospital, Peking University (Beijing, China).
N, N-dimethylethylenediamine (99%) and cholesteryl chloroformate (97%) were obtained from ACROS (USA); dipalmitoylphosphatidylcholine (DPPC) and polyethylene glycol-distearoylphosphatidylethanolamine (PEG-DSPE) were purchased from NOF (Tokyo, Japan); SG was generously supplied by Ryukakusan Co. Ltd. (Tokyo, Japan); cholesterol (Ch) was purchased from Wako Pure Chemical Industries (Tokyo, Japan); 3H-Ch and 125I-ODN was provided by China Institute of Atomic Energy (Beijing, China). FS was obtained from the Third Chemical Reagent Factory of Shanghai (Shanghai, China); DMEM medium and fetal bovine serum (FBS) was purchased from Life Technologies (NY, USA). 2, 5-Diphenyloxazole (PPO) and 1, 4-bis (5-phenyl-2-oxazolyl)-benzene (POPOP) were provided by Fluka (Buchs, Switzerland). Collagenase (II) was purchased from Sigma (St. Louis, MO, USA). All other chemicals were of reagent grade.
DC-chol was synthesized according to the method described by Gao[21]. The production was confirmed by thin-layer chromatogram (TLC), melting point, 1H nuclear magnetic resonance (NMR) (500 MHz, CDCl3), mass spectrum (MS), etc.
The FS or ODN encapsulated liposomes used in the present study were prepared according to the compositions in Table 1, respectively. A mixture of lipids in chloroform was dried under a stream of nitrogen and additionally dried under vacuum for 3 h to remove all chloroform. The dry lipid film was resuspended with FS or ODN solution (solution in 50 g/L glucose) by vortexing and sonicating, and then extruded through 0.2 μm pore size polycarbonate filters to generate the FS and ODN encapsulated liposomes, respectively. The concentration of lipids was 20 μmol/L. To prepare lipid-radioactive labeled liposomes, 3H-Ch was added to the lipid mixture at the beginning of the liposomes preparation and the dry lipid film was resuspended with 5% glucose. The 3H-labeled C-liposomes and C/SG/PEG-liposomes were prepared. The radioactivity of liposomes was 4 μCi/200 μL.
| Sample | Liposome compositions(molar ratio) | Entrapment of FS | Entrapment of ODN | ||||
| EE (%) | D (nm) | PI | E (%) | D (nm) | PI | ||
| N-liposomes | DPPC/Ch (10:10) | 0.64 | - | - | - | - | - |
| C-liposomes | DPPC/Ch/DC-chol (10:1:10) | 88.58±4.481 | 155 | 0.44 | 91.11±5.112 | 71 | 1 |
| C/SG-liposomes | DPPC/Ch/DC-chol/SG | ||||||
| (10:1:10:1.34) | 88.46±2.291 | 117.8 | 0.31 | - | 75.4 | 0.4 | |
| C/SG/PEG-liposomes | DPPC/Ch/DC-chol/SG/PEG- | ||||||
| DSPE (10:1:10:1.34:1.34) | 83.12±3.631 | 96.2 | 0.22 | 89.54±1.242 | 183 | 0.4 | |
Free ODN was separated from ODN encapsulated liposomes by equilibrium dialysis, in a dialysis tubing (SpectraPor 12 000 to 14 000 MWCO) at 4 °C for 12 h in 10 mL of 5% glucose solution. The incubation liquid was taken and the concentration of ODN was detected by UV spectro-photometer at 260 nm. Free FS was separated from encapsulated FS by passing through a Sephadex G-50 column (1 cm×20 cm). The concentration of FS was determined by measuring the fluorescence intensity of FS with excitation and emission wavelengths at 490 and 512 nm, respectively. According to the amount of ODN or FS entrapped in the liposomes, the encapsulation efficiency was calculated.
The size of liposomes was determined by dynamic light scattering using a Zetasier 3 000HS (Malvern Instruments, Ltd., UK). The morphologies of these liposomes were also observed by the transmission electron microscope.
HepG2 2.2.15 was maintained in DMEM medium supplemented with 100 mL/L FBS at 37 °C with 50 mL/L CO2. The cells were scraped by 0.25% trypsin and planted in 96-well tissue culture plates (5×103/well) for 2 d before the experiment, until the percentage of adherent cells reached approximately 70% confluence. The upper medium was removed and fresh DMEM medium was added with 100 mL/L FS encapsulated liposome. When the liposomes were incubated with cells for 3, 6, and 24 h, respectively, the cells were detached with 0.25% trypsin and washed thrice with 10 mmol/L PBS. The transfection efficiency was determined by counting the amount of cells transferred by FS with flow cytometry (BD, USA). The transfection efficiency was calculated according to the following equation: amount of FS transferred cells/amount of total cells’ 100%. The means of transfection efficiency were calculated from two independent experiments.
For lipofection, HepG2 2.2.15 cells were seeded at an initial concentration of 1×104 per well for 96-well plates. The cells were allowed to grow for about 24 h, until the percentage of adherent cells reached approximately 80% confluence. Then the cells were washed extensively to remove the previously secreted HBsAg and HBeAg in the medium. After washing, 100 μL free ODN or C/SG/PEG-liposomes entrapped ODN with an ODN concentration at 1.25, 2.5 or 5.0 μmol/L, together with 100 μL DMEM containing 10% serum, were added. The secretion of HBsAg and HBeAg into the culture supernatants was measured daily for 3 d, using ELISA immunoassay kits. The means of HBsAg and HBeAg immunoassay measurements were calculated from two independent experiments.
Liposomes labeled with 3H-Ch (C-liposomes and C/SG/PEG-liposomes) were injected into the tail vein of three male mice with a dose of 200 μL/20 g. At 0.5 and 4 h after injection, the mice were killed. The liver tissue was collected and washed with saline. About 100 mg of liver samples were decolored in a solution, containing 200 μL HClO4 and 300 μL 60% H2O2. Radiation scintillation fluid was added and mixed thoroughly. The radioactivity (dpm) of samples was counted on a scintillation counter (Pharmacia WALAC 1410, Turku, Finland).
Mice were injected intravenously by tail vein with 3H-labeled C-liposomes and C/SG/PEG-liposomes, respectively. At 0.5 and 4 h after administration, the mice were anesthetized, and the liver was perfused via the portal vein with isotonic saline to remove the blood. Then the liver was excised, minced and digested in 0.5 g/L collagenase for 30 min at 37 °C. The suspended cells were filtered through cotton mesh sieves, followed by centrifugation at 500 r/min for 3 min. The pellets containing hepatocytes were washed thrice with saline solution by centrifugation at 500 r/min for 3 min. The supernatant containing non-parenchymal cells were similarly centrifuged and washed thrice at 1 500 r/min for 15 min. The radioactivity (dpm) of hepatocytes and non-parenchymal samples was counted on a scintillation counter, as described previously.
The entrapment efficiencies, size, polydispersity index of all kinds of FS or ODN encapsulated liposomes are shown in Table 1. The results indicated that FS could hardly be entrapped in the N-liposomes, with an entrapment efficiency only at 0.64%. By adding DC-chol to the N-liposomes, the entrapment efficiencies of FS and ODN were increased to more than 83%, when the charge ratio of anionic FS or ODN/cationic lipid was 1:1. The C-liposomes, C/SG-liposomes and C/SG/PEG-liposomes had small size (155.0, 117.8, and 96.2 nm for FS entrapped in liposomes, 71.0, 75.4, and 183.0 nm for ODN entrapped in liposomes, respectively). The polydispersity index indicated the uniformity in size distribution. Transmission electron micrographs of these liposomes showed spherical vesicles (data not shown).
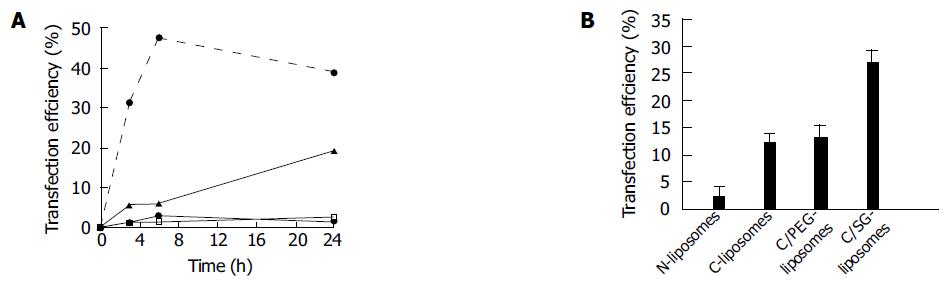
Figure 1 showed the transfection of FS entrapped in N-liposomes, C-liposomes and C/SG-liposomes when they were incubated for 3, 6, and 24 h with HepG2 2.2.15 cells at 37 °C, respectively. FS served as a fluorescent marker because the cell uptake of free FS was almost negligible. As shown in Figure 1A, the transfection of C-liposomes entrapped FS was significantly higher than that of N-liposomes, and the transfection of C/SG-liposomes entrapped FS was the highest. The enhancement of transfection efficiency was about 7.7-fold for the C/SG-liposomes than that of C-liposomes when incubated with cells for 6 h. When different types of liposomes were incubated with HepG2 2.2.15 cells at 37 °C for 24 h, the C/SG-liposomes showed the highest transfection efficiencies, while the C-liposomes and C/PEG-liposomes did not show significant differences (Figure 1B).
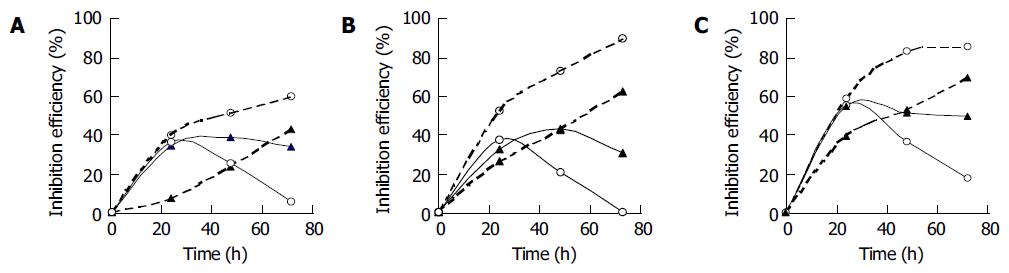
Figure 2 showed the effect of cellular treatment with free ODN and C/SG/PEG-liposomes entrapped ODN on the expression level of HBsAg and HBeAg protein in HepG2 2.2.15 cells. The antisense effect (percent of inhibition on HBsAg and HBeAg secretion) of free ODN and C/SG/PEG-liposomes entrapped ODN was affected by the concentration of ODN and the cultured times. When the concentration of ODN that was added increased from 1.25 to 5.0 μmol/L, the inhibition percentage of HBsAg by C/SG/PEG-liposomes entrapped ODN increased from 59.15% to 90.37% at 72 h, meanwhile, the inhibition of HBeAg increased from 42.9% to 73.43%. The inhibition effects of free ODN on HBsAg and HBeAg were decreased and the inhibition effects of C/SG/PEG-liposomes entrapped ODN were increased when incubation time was increased from 24 to 72 h. The inhibition on HBeAg secretion brought by free ODN and C/SG/PEG-liposomes entrapped ODN showed lower tendency compared to the HBsAg. The cells remained viable throughout the experiments and no morphological abnormalities were observed.
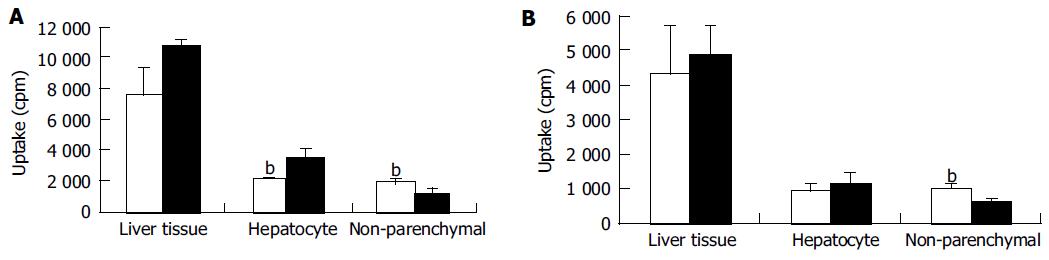
3H-Ch labeled C-liposomes and C/SG/PEG-liposomes were injected in mice at a dose of 4 μCi/20 g. At 0.5 and 4 h after injection, the radioactivity in 100 mg of liver tissue is shown in Figure 3. The distribution amount in liver tissue (total hepatocyte and non-parenchymal cells) was not significantly different for C-liposomes and C/PEG/SG-liposomes. The uptake amounts of liposomes at 0.5 and 4 h in hepatocytes and non-parenchymal cells are also shown in Figure 3. After separating liver cells into hepatocytes and non-parenchymal cells, it was found that the uptake of C/SG/PEG-liposomes was higher than that of C-liposomes by hepatocytes at 0.5 h (P<0.01) and the uptake of C/SG/PEG-liposomes was lower than that of C-liposomes by non-parenchymal cells at 0.5 and 4 h (P<0.01). These results indicated that the C/SG/PEG-liposomes have more appetency to hepatocytes than non-parenchymal cells in the liver.
Among various types of non-viral vector systems, cationic liposomes seem to be promising, because of their high gene expression efficiency. When using simple cationic liposomes by both intravenous and intraportal administration[19,22], it is difficult to transfect into hepatocytes because liposomes prefer targeting the lung and RES. A great challenge faces the investigator who wishes to target liposomes to hepatocytes, for some disorders such as hepatitis B or metabolic diseases which require that the liposomes be steered away from their natural targets. In this study, the characteristics of SG modified liposomes and its inhibition on HBsAg and HBeAg secretion was investigated. In addition, the liver uptake and intrahepatic distribution of a labeled cationic liposome and SG modified cationic liposome were also evaluated.
To investigate the effect of DC-chol, SG and PEG-DSPE on the entrapment and transfection efficiency, FS was used as a marker. DC-chol (positively charged) can bind with FS or ODN (both negatively charged) by electrostatic interaction. Typical cationic liposomes that carry excess positive charge will interact with plasma proteins, and would be rapidly taken up into the mononuclear phagocytic system. In order to decrease the adsorption of plasma proteins and interaction with non-target cells, 6% PEG-DSPE was added into the cationic liposomes.
Entrapment of FS and ODN to cationic liposomes was very efficient, even when containing a relatively high amount of SG and PEG-DSPE (6%, Table 1). Evidently, SG and PEG coating seldom shields the positively charged liposome surface from interaction with FS and ODN.
Receptors for carbohydrates such as the ASGP-R on hepatocytes and the mannose receptor on macrophages and liver endothelial cells produce opportunities for cell-specific gene delivery with liposomal carriers. The presence of a glucoside on the surface of electrically neutral FS entrapped in C/SG-liposomes, resulted in more than a 2-fold increased transfection efficiency of HepG2 cells, which is a human hepatoblastoma cell line that is known to express ASGP-R, when compared to C-liposomes encapsulated FS (Figure 1). It was surmised that such glucoside in SG could be identified by ASGP-R present on the surface of the HepG2 cells, leading to liposome entry into cells through endocytosis.
The major HBsAg, and in some cases, the HBeAg, is detectable in the serum of individuals with chronically infected HBV. Serological detection of HBeAg usually correlates well with the presence of circulating virions and is commonly used clinically as an indicator of active HBV replication. Elimination of HBsAg and HBeAg from the supernatant is associated with resolution of infection with a wild-type strain of HBV. Synthetic ODN (15-mers), complementary to the cap site of SP II promoter transcribed mRNA, showed a sequence-specific, dose-dependent inhibitory effect on HBV gene expression from a concentration of 1.25-5.0 μmol/L. A previous study showed that, such ODN could inhibit the expression of HBsAg without a significant effect on total synthesized protein in the cells[23]. According to the results of this study, it is found that the initial concentration of ODN and incubation time appeared to be important factors in obtaining a desired inhibition effect. As the ODN concentration and incubation time increased, the inhibition efficiency of C/SG/PEG-liposomes entrapped ODN also increased (Figure 2). However, it is demonstrated that larger amounts of ODN and cationic lipid would lead to a higher toxicity to the cells; therefore, concentration of liposome-ODN complexes should be restricted at a certain level[24]. As for the present ODN encapsulated C/SG/PEG-liposomes, a concentration of DC-chol lower than 10 μg/mL with a cationic lipid/ODN at 1:1 of charge ratio was regarded suitable.
In the study of liver uptake of 3H labeled cationic liposomes, no significant difference was found between C-liposomes and C/SG/PEG-liposomes. These results indicated that the PEG chain and glucose residue in SG did not necessarily improve the in vivo behavior of cationic liposomes. Kirby et al[22,25] showed that when the cationic liposomes contain only 5% of charged lipid with small zeta potential, the behavior of the cationic liposomes are not different from that of neutral liposomes. Our results agree with these findings. These results could be attributed to the special hepatocytes targeting behavior of SG. A previous study showed that the glucose residue on the surface of liposomes could selectively recognize the ASGP-R of hepatocytes cells[9]. The result also demonstrated that the liver targeting effect of SG is not diminished by the PEG chain on the surface of liposomes.
In conclusion, this study established a highly efficient receptor-mediated delivery system for ODN to hepatocytes by using SG and PEG modified cationic liposomes. By utilizing this delivery system, an ODN targeting the encapsidation site of the HBV pregenome causes a strong inhibition of HBV replication in vitro. Therefore, SG modified liposomes may be effective vehicles to improve the delivery of ODN to the liver for the therapy of hepatotropic viruses.
We would like to thank Professor Lai Wei and his research group at the Institute of Hepatology, Peking University for assistance in cell culture and transfection efficiency measurement.
| 1. | Zhu N, Liggitt D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 532] [Article Influence: 16.1] [Reference Citation Analysis (5)] |
| 2. | Huang L, Li S. Liposomal gene delivery: a complex package. Nat Biotechnol. 1997;15:620-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Dasgupta P, Bachhawat BK. Receptor-mediated uptake of asialoganglioside liposomes: sub-cellular distribution of the liposomal marker in isolated liver cell types. Biochem Int. 1985;10:327-336. [PubMed] |
| 4. | Hara T, Aramaki Y, Takada S, Koike K, Tsuchiya S. Receptor-mediated transfer of pSV2CAT DNA to a human hepatoblastoma cell line HepG2 using asialofetuin-labeled cationic liposomes. Gene. 1995;159:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Remy JS, Kichler A, Mordvinov V, Schuber F, Behr JP. Targeted gene transfer into hepatoma cells with lipopolyamine-condensed DNA particles presenting galactose ligands: a stage toward artificial viruses. Proc Natl Acad Sci USA. 1995;92:1744-1748. [RCA] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Choi YH, Liu F, Park JS, Kim SW. Lactose-poly(ethylene glycol)-grafted poly-L-lysine as hepatoma cell-tapgeted gene carrier. Bioconjug Chem. 1998;9:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Kircheis R, Kichler A, Wallner G, Kursa M, Ogris M, Felzmann T, Buchberger M, Wagner E. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997;4:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 253] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Wagner E. Application of membrane-active peptides for nonviral gene delivery. Adv Drug Deliv Rev. 1999;38:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Shimizu K, Maitani Y, Takayama K, Nagai T. Evaluation of dipalmitoylphosphatidylcholine liposomes containing a soybean-derived sterylglucoside mixture for liver targeting. J Drug Target. 1996;4:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Shimizu K, Maitani Y, Takayama K, Nagai T. Formulation of liposomes with a soybean-derived sterylglucoside mixture and cholesterol for liver targeting. Biol Pharm Bull. 1997;20:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Shimizu K, Qi XR, Maitani Y, Yoshii M, Kawano K, Takayama K, Nagai T. Targeting of soybean-derived sterylglucoside liposomes to liver tumors in rat and mouse models. Biol Pharm Bull. 1998;21:741-746. [PubMed] |
| 12. | Shimizu K, Maitani Y, Takayama K, Nagai T. Characterization of dipalmitoylphosphatidylcholine liposomes containing a soybean-derived sterylglucoside mixture by differential scanning calorimetry, Fourier transform infrared spectroscopy, and enzymatic assay. J Pharm Sci. 1996;85:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Maynard JE. Hepatitis B: global importance and need for control. Vaccine. 1990;8 Suppl:S18-20; discussion S21-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 216] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Hoofnagle JH. Chronic hepatitis B. N Engl J Med. 1990;323:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Dusheiko GM. Treatment and prevention of chronic viral hepatitis. Pharmacol Ther. 1995;65:47-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Goodarzi G, Gross SC, Tewari A, Watabe K. Antisense oligodeoxyribonucleotides inhibit the expression of the gene for hepatitis B virus surface antigen. J Gen Virol. 1990;71:3021-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Wu GY, Wu CH. Specific inhibition of hepatitis B viral gene expression in vitro by targeted antisense oligonucleotides. J Biol Chem. 1992;267:12436-12439. [PubMed] |
| 18. | Offensperger WB, Offensperger S, Walter E, Teubner K, Igloi G, Blum HE, Gerok W. In vivo inhibition of duck hepatitis B virus replication and gene expression by phosphorothioate modified antisense oligodeoxynucleotides. EMBO J. 1993;12:1257-1262. [PubMed] |
| 19. | Soni PN, Brown D, Saffie R, Savage K, Moore D, Gregoriadis G, Dusheiko GM. Biodistribution, stability, and antiviral efficacy of liposome-entrapped phosphorothioate antisense oligodeoxynucleotides in ducks for the treatment of chronic duck hepatitis B virus infection. Hepatology. 1998;28:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994;372:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 565] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991;179:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 639] [Article Influence: 18.3] [Reference Citation Analysis (6)] |
| 22. | Ross PC, Hui SW. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999;6:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 203] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 357] [Article Influence: 12.3] [Reference Citation Analysis (11)] |
| 24. | Allen TM, Austin GA, Chonn A, Lin L, Lee KC. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochim Biophys Acta. 1991;1061:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 267] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Huang SK, Martin FJ, Friend DS, Papahadjopoulos D. Mechanism of stealth liposome accumulation in some pathological tissues, In: D. Lasic, F. Martin (Eds.), Stealth Liposomes, Boca Raton CRC Press FL 1995. 119-125. |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK