Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3426
Revised: July 3, 2004
Accepted: September 9, 2004
Published online: June 14, 2005
AIM: To establish a model of islet-ductal cell transdifferen-tiation to identify the transdifferentiated cells.
METHODS: Collagen was extracted from rat tail at first. Purified rat islets were divided into three groups, embedded in collagen gel and incubated respectively in DMEM/F12 alone (control group), DMEM/F12 plus epidermal growth factor (EGF), DMEM/F12 plus EGF and cholera toxin (CT). Transdifferentiation was proved by microscopy, RT-PCR, immunohistochemistry and RIA.
RESULTS: Islets embedded in collagen gel plus EGF and CT were cystically transformed and could express new gene cytokeratin 19 while still maintaining the expression of insulin and Pdx-1 genes. Immunohistochemistry demonstrated that the protein of cytokeratin 19 was only expressed in the third group. The insulin content secreted by islets in the third group decreased significantly during the transdiffe-rentiation.
CONCLUSION: CT is a crucial factor for the islet-ductal cell transdifferentiation.
- Citation: Lu J, Gu YP, Xu X, Liu ML, Xie P, Song HP. Adult islets cultured in collagen gel transdifferentiate into duct-like cells. World J Gastroenterol 2005; 11(22): 3426-3430
- URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3426.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3426
Transdifferentiation is a process in which differentiated cells alter their identity to become other distinct cell types. This process has been proven by researches[1,2]. During pancreas development, both the exocrine and endocrine systems seem to originate from the ductal cells which are considered as a kind of pancreatic stem cells. This hypothesis is supported by the phenomenon that ductal cells have the ability to transdifferentiate into acinar cells and islets[3-5]. But this process is bilateral. On the contrary, acinar cells and islets can transdifferentiate into duct-like cells[6,7]. It has also been reported that islets in a long-term culture can transdifferentiate into exocrine and undifferentiated cells, which may be considered as pancreatic precursor cells[8]. The process of mature endocrine cells transdifferentiating into exocrine cells was also confirmed in our study. Our data show that, rat islets cultured in rat tail collagen gel plus EGF and CT can transdifferentiate into duct-like cells.
Male Sprague-Dawley (SD) rats, weighing 230-250 g, were obtained from Central South University Laboratory Animal Department. For each isolation, one rat was anesthetized with pentobarbital sodium (30 mg/kg) intraperitoneally. Isolation of pancreatic islets was performed as previously described[9]. Briefly, after the output site of the duct from pancreas to duodenum was clamped, the pancreas was distended maximally with 20 mL collagenase (type IV, 1.5 mg/mL, Invitrogen) using a pulsed infusion technique via the portal duct of pancreas. The whole pancreas was removed from male SD rats and placed in a stationary water bath at 37 °C for 25 min to allow it to digest enzymatically. Digestate was agitated gently for 1 min every 5 min during incubation. The digestion process was stopped by adding 30 mL of cold Hank’s balanced salt solution (HBSS) supplemented with 5% fetal bovine serum (FBS). After having vortexed for 1 min to dissociate the islets from adherent acinar elements, the mixture was passed through a steel mesh filter with an aperture of 0.5 mm. The filtrate was subjected to the conventional method of histopaque density gradient centrifugation to separate islets from the digestion mixture.
DTZ stock solution preparation Ten milligrams of DTZ were dissolved entirely in 5 mL of dimethyl sulfoxide (DMSO), then filtered through a 0.2-µm nylon filter and stored briefly at -20 °C.
In vitro DTZ staining Ten microliters of the stock solution was added to 1 mL of culture medium. The culture dishes were incubated at 37 °C for 15 min in the DTZ solution. After the dishes were rinsed thrice with HBSS, clusters stained crimson red were identified as islets. These islets were hand-picked under a stereomicroscope. After hand-picking, the dishes were refilled with DMEM containing 10% FBS. The stain completely disappeared from the cells after 2 h.
Collagen preparation Collagen was extracted from rat tail tendons as previously described[10]. Tendons were excised from rat tails and sheared. Some connective tissues were removed carefully, washed twice with PBS and then soaked in 4 mmol/L acetic acid. After having stirred for two d at 4 °C, collagen was extracted. The extracts were centrifuged for 30 min at 10000 g and supernatant was collected into another distilled vessel for use. Protein concentration in the collagen was 1.0-1.5 mg/mL measured with an ultraviolet spectrophotometer.
Preparation of collagen gels Following sterile components (0.7 mL 10×DMEM/F12, 0.1 mL heat-inactivated low-endotoxin FCS, 0.1 mL 0.1 mol/L NaOH, 0.1 mL islet suspension) were carefully mixed to avoid bubbles. All components required to make collagen gels were placed on ice except for the islet suspension. Thus, the mixture resulted in a physiologic ionic strength and 1×DMEM/F12 in the final gel. The mixture was incubated at 37 °C in 50 mL/L CO2 and then gelation was completed after 60 min.
Islet culture Islets embedded in collagen gel were divided into three groups and incubated under the following conditions.
3D culture alone (control group) DMEM/F12 (penicillin 100 U/mL, streptomycin 100 μg/mL, 10 mmol/L HEPES)+10% FCS+nicotinamide (10 mmol/L).
3D culture+EGF: DMEM/F12 (penicillin 100 U/ml, streptomycin 100 μg/mL, 10 mmol/L HEPES)+10% FCS +EGF (100 ng/mL)+nicotinamide (10 mmol/L).
3D culture + EGF + CT: DMEM/F12 (penicillin 100 U/mL, streptomycin 100 μg/mL, 10 mmol/L HEPES)+10% FCS+EGF (100 ng/mL)+CT (100 ng/mL)+nicotinamide (10 mmol/L).
Samples were taken at the 1st, 3rd, 5th, 16th, 28th, 40th, 52nd, 76th, 88th,120th, 168th h. After old culture medium was piped out thoroughly, islets were rinsed twice and fresh medium was added. Culture media were sampled after 1 h and frozen at -20 °C. Insulin content in samples was detected by radioimmunoassay (RIA).
Islets were hand-picked from slides pretreated with poly-L-lysine and fixed in 4% paraformaldehyde (PFA). Then the fixed islets were processed for routine histology and immun-ostained for insulin (mouse anti-insulin, Boster, Wuhan) and cytokeratin 19 (mouse anti-cytokeratin 19, Boster, Wuhan) using the streptavidin-biotin complex (SABC) method following the instructions of kit (Boster, Wuhan). For cytokeratin 19, islet cells were pretreated with 0.1% trypsin. The sections were incubated overnight at 4 °C with appropriate primary antibodies. Negative controls involved omission of the primary antibodies.
The collagen matrix was dissolved in 0.25 mg/mL collagenase and islets were washed thrice. Total RNA was extracted from cultured rat islets using TRIzol (GIBCO BRL) and reverse-transcribed into cDNA with TaKaRa RNA PCR kit (AMV) Ver. 2.1 (TaKaRa, Japan) according to the standard procedure. cDNA samples were subjected to PCR amplification with specific primers. The cycling parameters were pre-denaturation at 94 °C for 2 min, denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 1 min (34 cycles). Table 1 summarizes the sequences of the PCR primers used in this study.
| Targeted mRNA | Primer | Length |
| Rat Pdx-1 | F: gaggacccgtacagcctaca | 201 bp |
| R: cgttgtcccgctactacgtt | ||
| Rat Insulin | F: ccgtcgtgaagtggagga | 154 bp |
| R: cagttggtagagggagcagat | ||
| Rat Cytokeratin 19 | F: atccccaaagacacgagatg | 200 bp |
| R: gtgagctacaaccgcagctt | ||
| Rat β-actin | F: taaagagaagctgtgctatgttgc | 354 bp |
| R: atgatcttgatcttcatggtgcta |
All data were expressed as mean±SD with n = 3 at each time point. The difference between time points with respect to insulin content was evaluated by one-way ANOVA. P<0.05 was considered statistically significant.
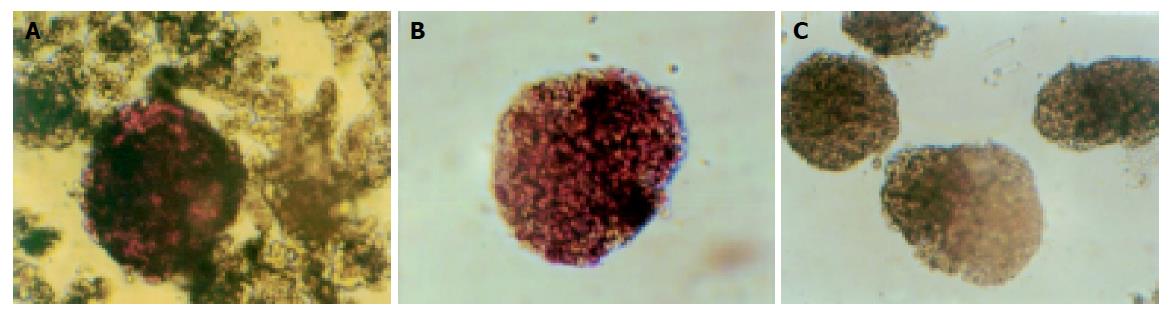
We used DTZ to identify islets which were stained crimson while acinar and ductal tissue failed to incorporate the stain. Islets were stained with DTZ and hand-picked after density gradient centrifugation. The stain completely disappeared from the cells after 2 h (Figure 1).
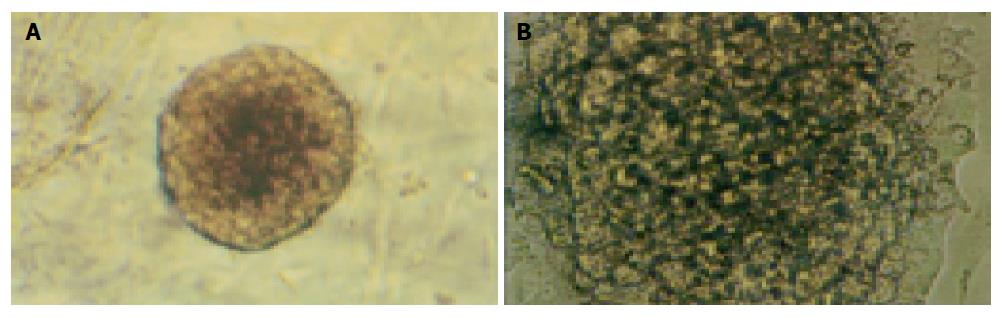
Islets embedded in collagen gel could retain their natural shape, while those in monolayer cultures could not (Figure 2).
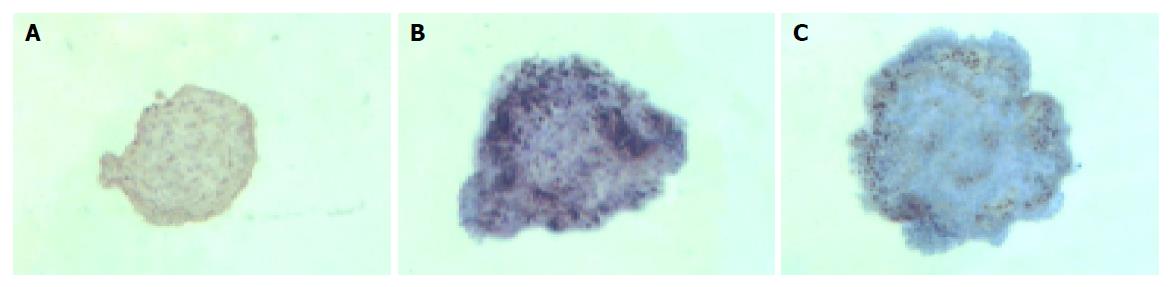
The islets in groups 1 and 2 were not different in shape on d 1, 3 and 5. But some islets in group 3 continually enlarged in these days (Figure 3). The percentage of islets undergoing cystic transformation was increased over the time-course of the culture period in group 3.
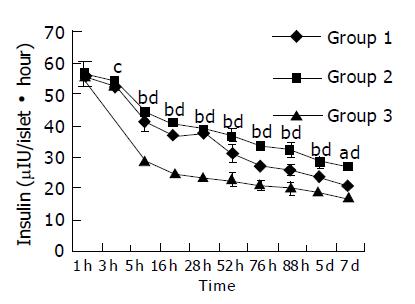
During the culture time, the abilities of islets to secrete insulin in three groups were all decreased. Five hours later, islets lost their secreting ability faster in group 3 than in groups 1 and 2 (P<0.05 or 0.01) (Figure 4).
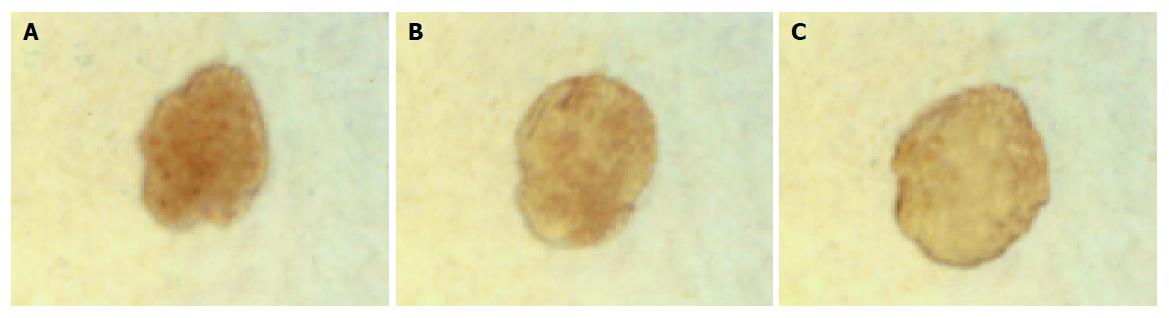
Islets cultured for 1, 3, 5 d were insulin positive and cytokeratin 19 negative in groups 1 and 2.
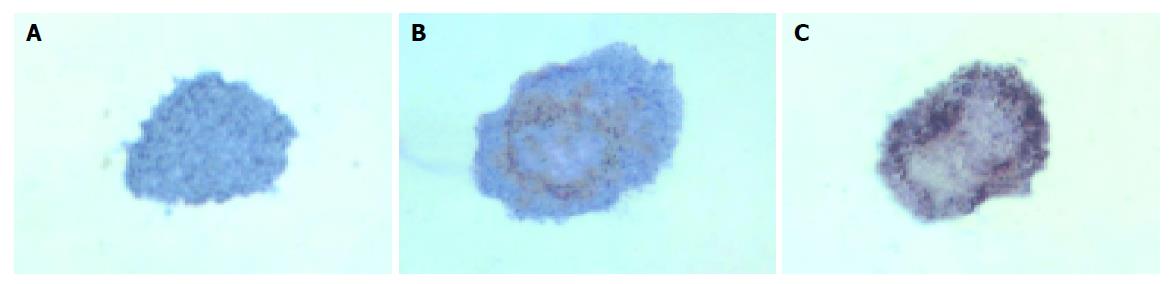
In group 3, islets were insulin positive and cytokeratin 19 negative on d 1. There was a decrease in expression of insulin mainly in the center of islets and some islet cells around the cystic spaces began to express protein cytokeratin 19 on d 3. Only a few cells in the islets expressed insulin continually on d 5, but most cells expressed cytokeratin 19 (Figures 5 and 6).
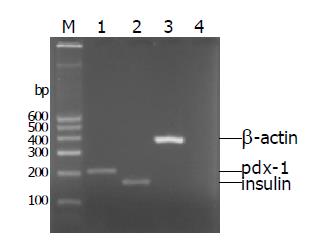
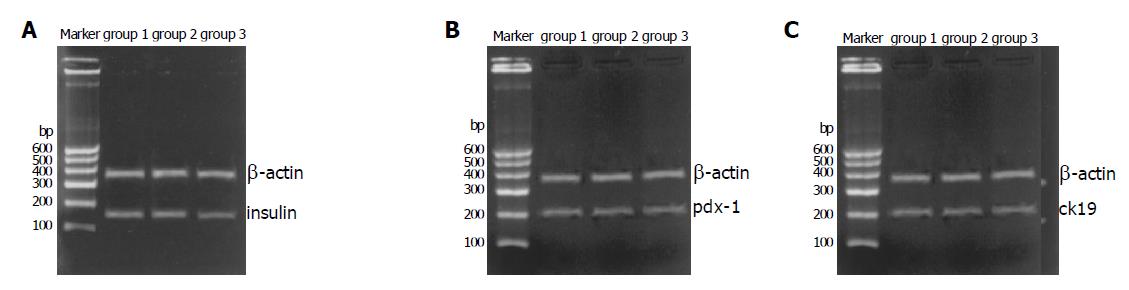
Freshly isolated islets expressed islet characteristic genes (pdx-1 and insulin) but did not express duct characteristic gene (cytokeratin 19) (Figure 7). After incubation for 7 d, islets in the three groups could express genes (insulin and pdx-1), but cytokeratin 19 was only expressed in group 3 (Figure 8).
Cells of both the exocrine (acinar cells) and endocrine systems (islet cells) seem to originate from the ductal cells. From experiments in vitro it is evident that during development, endocrine cells emerge from the pancreatic ducts and form aggregates that eventually form islets of Langerhans. Teitelman et al[11], removed pancreatic rudiments from E11 mouse embryos and maintained in culture, and found that pancreas (including exocrine and endocrine tissues) regenerates in vitro from E11 pancreatic ducts. Rosenberg et al[12], reported that partial obstruction of the pancreatic duct in adult hamsters leads to islet cell differentiation from cells in the interlobular ducts, followed by formation of new islets. More recently, Susan Bonner-Weir et al[5], cultivated human adult pancreatic duct cells in vitro and differentiated the duct cells into “cultivated human islet buds”(CHIBs) which have the ability to secrete glucose-response insulin.
On the other hand, islets can transdifferentiate into duct-like cells. Human and dog islets embedded in collagen gel and cultured in media plus EGF and CT for several days can transform and express duct cell marker CK-19[7,13]. In this study, we isolated rat islets and incubated them in the same conditions as above, and proved that rat islets could transdifferentiate into duct-like cells.
Extracellular matrix (ECM) is one of the most important components in creating cellular microenvironment. Among the ECM components, collagen can provide cells with a bio-mimic environment favorable for their reorganization, or maintenance of the three-dimensional structure. The use of collagen gel as an extracellular matrix material is an important part of the culture system in our study. Collagen gel matrix can help promote or maintain the differentiated state of cells in culture, such as liver cells[14] and mammary epithelial cells[15]. ECM may also promote the process of cell transdifferentiation[6,16]. Collagen plays an important role in the process. Islets cannot transdifferentiate in agarose gel[13]. ECM is the necessary condition for transdifferentiation. In our study, islets embedded in collagen gel without EGF and CT did not express CK-19, consistent with the report by Wang et al[13].
EGF can activate protein tyrosine kinase (PTK), protein kinase C and increase intracellular Ca2+ concentration after binding to EGF receptor[17]. CT can activate adenylate cyclase, and result in the increase of intracellular cAMP concentration[18]. It was reported that the process of cystic transformation requires both an elevation of intracellular cAMP and the presence of ECM proteins[13]. Both cAMP and Ca2+ are classic second messengers. Perhaps EGF and CT affect the signal transduction of islet cells in two ways by increasing the concentration of these messengers. In our study, we found that EGF alone had no ability to initiate islet cell transdifferentiation, but it could preserve islet’s function. It is supported by the fact that both EGF and betacellulin are members of EGF-family of cytokines, and betacellulin is a kind of beta-cell growth factor, suggesting that EGF also can stimulate beta-cell growth.
In conclusion, the process of transdifferentiation is promoted by cooperation of collagen matrix, EGF and CT. But the exact mechanisms of interaction of these factors remain to be fully elucidated.
| 1. | Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Mu W, Nikolic-Paterson DJ, Atkins RC, Lan HY. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;54:864-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 300] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 362] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 356] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Walker NI, Winterford CM, Kerr JF. Ultrastructure of the rat pancreas after experimental duct ligation. II. Duct and stromal cell proliferation, differentiation, and deletion. Pancreas. 1992;7:420-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999-8004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 738] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Arias AE, Bendayan M. Differentiation of pancreatic acinar cells into duct-like cells in vitro. Lab Invest. 1993;69:518-530. [PubMed] |
| 7. | Yuan S, Rosenberg L, Paraskevas S, Agapitos D, Duguid WP. Transdifferentiation of human islets to pancreatic ductal cells in collagen matrix culture. Differentiation. 1996;61:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Schmied BM, Ulrich A, Matsuzaki H, Ding X, Ricordi C, Weide L, Moyer MP, Batra SK, Adrian TE, Pour PM. Transdifferentiation of human islet cells in a long-term culture. Pancreas. 2001;23:157-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Inoue K, Gu Y, Shinohara S, Kogire M, Mitsuo M, Nakai I, Hayashi H, Uchida K, Maetani S, Ikada Y. Isolation of adult pig islet. In vitro assessment and xenotransplantation. Int J Pancreatol. 1992;12:173-180. [PubMed] |
| 10. | Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996;32:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Teitelman G, Lee J, Reis DJ. Differentiation of prospective mouse pancreatic islet cells during development in vitro and during regeneration. Dev Biol. 1987;120:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Rosenberg L, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983;35:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Wang R, Li J, Rosenberg L. Factors mediating the transdifferentiation of islets of Langerhans to duct epithelial-like structures. J Endocrinol. 2001;171:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Rubin K, Höök M, Obrink B, Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981;24:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Yang J, Larson L, Flynn D, Elias J, Nandi S. Serum-free primary culture of human normal mammary epithelial cells in collagen gel matrix. Cell Biol Int Rep. 1982;6:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 125] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P. The epidermal growth factor. Cell Biol Int. 1995;19:413-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 225] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 387] [Article Influence: 8.6] [Reference Citation Analysis (0)] |