Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3431
Revised: December 21, 2004
Accepted: January 5, 2005
Published online: June 14, 2005
AIM: Recent reports have shown the capacity of mesenchymal stem cells (MSCs) to differentiate into hepatocytes in vitro and in vivo. MSCs administration could repair injured liver, lung, or heart through reducing inflammation, collagen deposition, and remodeling. These results provide a clue to treatment of liver fibrosis. The aim of this study was to investigate the effect of infusion of bone marrow (BM)-derived MSCs on the experimental liver fibrosis in rats.
METHODS: MSCs isolated from BM in male Fischer 344 rats were infused to female Wistar rats induced with carbon tetrachloride (CCl4) or dimethylnitrosamine (DMN). There were two random groups on the 42nd d of CCl4:CCl4/MSCs, to infuse a dose of MSCs alone; CCl4/saline, to infuse the same volume of saline as control. There were another three random groups after exposure to DMN: DMN10/MSCs, to infuse the same dose of MSCs on d 10; DMN10/saline, to infuse the same volume of saline on d 10; DMN20/MSCs, to infuse the same dose of MSCs on d 20. The morphological and behavioral changes of rats were monitored everyday. After 4-6 wk of MSCs administration, all rats were killed and fibrosis index were assessed by histopathology and radioimmunoassay. Smooth muscle alpha-actin (alpha-SMA) of liver were tested by immunohistochemistry and quantified by IBAS 2.5 software. Male rats sex determination region on the Y chromosome (sry) gene were explored by PCR.
RESULTS: Compared to controls, infusion of MSCs reduced the mortality rates of incidence in CCl4-induced model (10% vs 20%) and in DMN-induced model (20-40% vs 90%).The amount of collagen deposition and alpha-SMA staining was about 40-50% lower in liver of rats with MSCs than that of rats without MSCs. The similar results were observed in fibrosis index. And the effect of the inhibition of fibrogenesis was greater in DMN10/MSCs than in DMN20/MSCs. The sry gene was positive in the liver of rats with MSCs treatment by PCR.
CONCLUSION: MSCs treatment can protect against experimental liver fibrosis in CCl4-induced or DMN-induced rats and the mechanisms of the anti-fibrosis by MSCs will be studied further.
- Citation: Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol 2005; 11(22): 3431-3440
- URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3431.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3431
Liver fibrosis is the wound-healing response of the liver to chronic injury[1]. Following repeated injury, the liver undergoes a tissue remodeling and forms fibrosis. It is characterized by excessive accumulation of extracellular matrix, with the formation of scar tissue encapsulating the area of injury. This results in many clinical manifestations, including ascites, variceal hemorrhage, and encephalopathy. The prognosis of patients with the disease is poor, although liver transplantation is a good alternative treatment. Moreover, there are limited available donor livers for hundreds of millions of patients worldwide[2,3]. So, it is very important to investigate appropriate therapies for the disease by different treatments.
We[4] and others[5-9] have established that BM-derived MSCs could engraft injured tissue, such as, bone marrow, lung, liver, heart or brain, and recover its function. These results indicate that MSCs is an attractive cell source for regenerative medicine. Recent reports have shown the capacity of MSCs to differentiate into hepatocytes in vitro[10,11,] and in vivo[6,12,13]. Further, MSCs administration could repair injured lung, liver, or heart by reducing inflammation, collagen deposition and remodeling[5-7]. These imply that MSCs could not only have a possibility to repair acute damaged tissue but also have potentiality of reducing chronic fibrogenesis. Although Sakaida et al[14], and Fang et al[6], reported that BM or MSCs could reduce CCl4-induced liver fibrosis in mice, the mechanism by which MSCs repair the fibrosis is unclear and their results seem controvertible. Fang et al[6], suggested that delaying MSCs administration by 1 wk after CCl4 challenge did not prevent the disease progression, while Sakaida et al[14], found that BM treatment to 4 wk CCl4-induced rats could get significantly reduced liver fibrosis. From the point of view of clinical practice, it is very important to select the model of liver fibrosis close to human disease for evaluating the effect of MSCs on the fibrosis. In the present study, we employed two models of fibrosis induced with CCl4 or DMN to evaluate the effect of MSCs on the fibrosis. Our results showed that MSCs treatment could reduce mortality rates of rats and improve their fibrosis index, and earlier the MSCs were administrated, greater was the anti-fibrosis effect. We provide evidence here indicating that infusion of BM-derived MSCs should be therapeutic in liver fibrosis by blocking the fibrosis formation and progression.
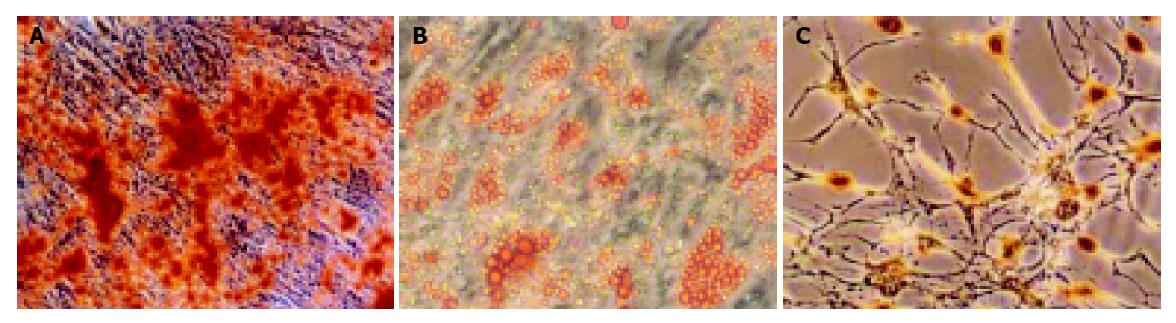
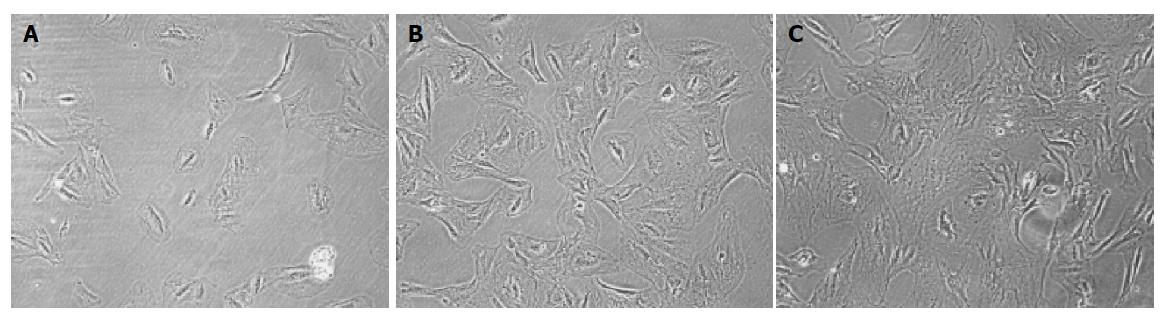
MSCs were prepared from rat bone marrow as described previously in our laboratory[15]. In brief, whole BM was flushed from the tibia and femur of Fischer 344 rats (6 wk old, male). MSCs preferentially attached to the polystyrene surface and were further purified by passages. MSCs were identified to express CD29 but not MHC class II antigens, costimulatory molecules CD80 and CD86 by flow cytometry[15]. The multiple differentiation potential of MSCs into neural cells, adipocyte and osteoblasts was also confirmed in vitro.
The multiple differentiation potential of MSCs into neural cells, adipocyte, and osteoblasts was also confirmed in vitro. In brief, to induce osteogenic differentiation, 8th-10th-passage cells were treated with osteogenic medium for 3 wk with medium changes twice weekly. Osteogenic medium consists of L-DMEM supplemented with 0.1 μmol/L dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 10 mmol/L β-glycerol phosphate (Sigma-Aldrich), and 0.2 mmol/L ascorbic acid (Sigma-Aldrich). Osteogenesis was assessed by Chinalizarin staining. To induce adipogenic differentiation, 8th-10th-passage cells were treated with adipogenic medium for 3 wk. Medium changes were performed twice weekly. Adipogenic medium consists of H-DMEM supplemented with 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 1 μmol/L hydrocortisone (Sigma-Aldrich), 0.1 mmol/L indomethacin (Sigma-Aldrich), and 10% rabbit serum (Sigma-Aldrich). Adipogenesis was assessed by Oil Red O staining. To induce neurogenic differentiation, 8th-10th-passage cells were pretreated for 24 h with neurogenic medium, which consists of H-DMEM supplemented with 1 mmol/L β-mercaptoethanol (β-ME, Sigma-Aldrich). Then cells were treated with 5 mmol/L β-ME for 1 h. Neurogenesis was identified by antibody against neuron-specific enolase (NSE, SouthernBiotech, Birmingham, USA) staining.
Fischer 344 rats BM-MSCs in the 8-10th passage were suspended in sterile saline and used for further experiments as described below.
Female Wistar rats were 6-wk old, weighing between 140 and 160 g. Rats were bred and maintained in an air-conditioned animal house with specific pathogen-free conditions, and were subjected to a 12:12-h daylight/darkness and allowed unlimited access to chow and water. The morphological and behavioral changes of rats were monitored everyday. Liver fibrosis was induced by CCl4 or DMN administration as described[16]. The protocol was approved by Animal Care Committee of Sun Yat-Sen University.
On d 0, rats were injected by subcutaneous at a dose of 0.2 mL/100 g body weight of 40 mL/L CCl4 (Guangzhou Chemical Factory, China) dissolved in paraffin oil (Guangzhou Chemical Factory, China). The injection was given twice a week for 6 wk. The same volume of paraffin oil alone was used as control. Liver fibrosis was determined by killing three to six rats with histopathology weekly. Twenty rats were randomly divided into two groups on d 42: CCl4/MSCs, n = 10, to infuse a dose of MSCs 3×106 cells per rat by intravenous; CCl4/saline, n = 10, to infuse the same volume of saline as a control. Blank control were designed, paraffin/saline, n = 10, to infuse the same volume of saline on d 42 after paraffin administration. On d 70, all rats were killed and venous blood was collected and liver tissue harvested for analysis.
On d 0, rats were injected intraperitoneally at a dose of 100 μL DMN (Sigma, USA) (diluted 1:100 with 0.15 mol/L sterile saline) per 100 g body weight. The injection was given on three consecutive days of each week for 6 wk. Control animals received the same volume of 0.15 mol/L sterile saline. Three or six treated animals were killed weekly with determination of establishment fibrosis by histopathology. Sixty rats were randomly divided into three groups in different time points of DMN-induced: DMN10/MSCs, n = 10, to infuse a dose of MSCs 3×106 cells per rat by intravenous on d 10; DMN20/MSCs, n = 10, to infuse the same dose MSCs on d 20; DMN10/saline, n = 40, to infuse the same volume of saline on d 10. We also designed saline, n = 10, to infuse the same volume of saline for blank control. On d 49, venous blood was collected and all rats were killed, and liver tissue harvested for analysis.
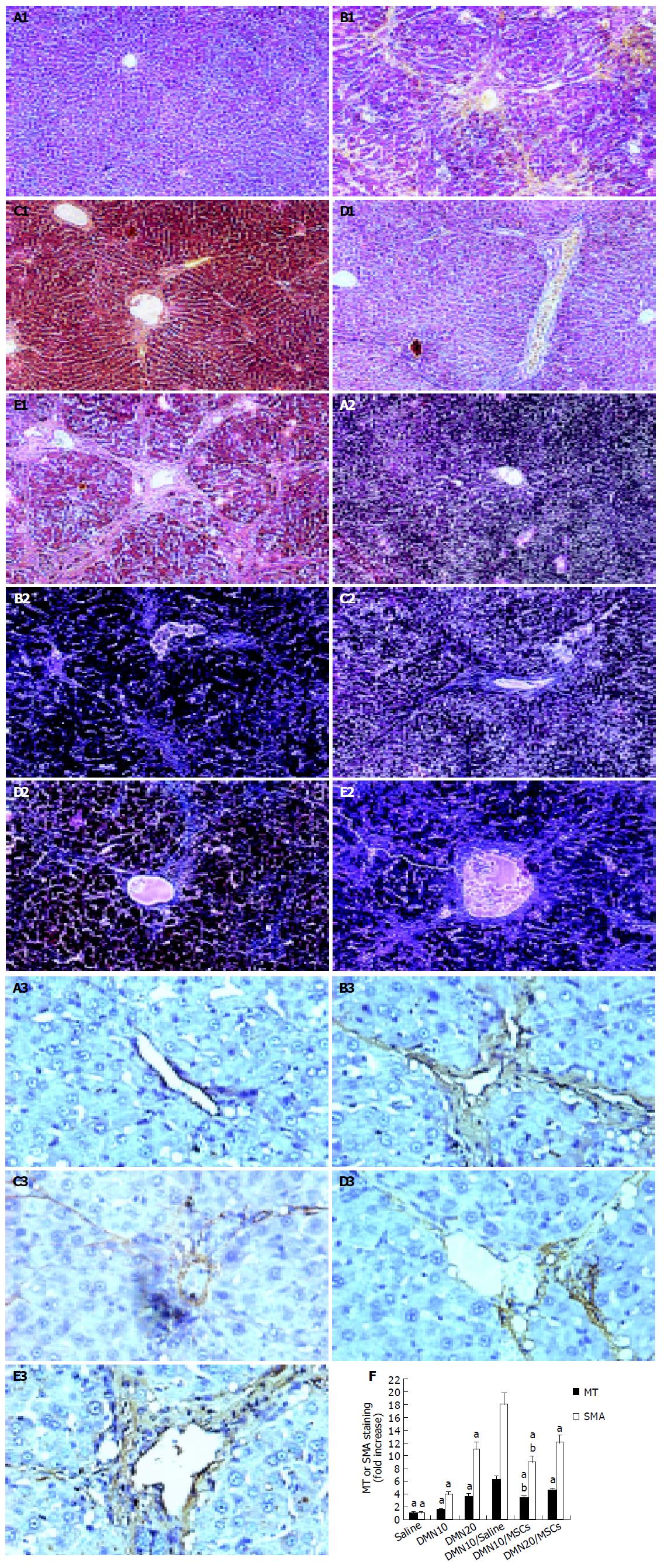
Liver samples were collected into PBS and fixed overnight in 40 g/L paraformaldehyde in PBS at 4 °C. Serial 5-μm sections of the right lobes of the livers were stained with hematoxylin and eosin (HE) and Masson Trichrome (MT) as rules in our laboratory. Additional sections were tested with the antibody against α-SMA (1:200 dilutions, NeoMarkers) and immunoreactive materials were visualized by use of a streptavidin-biotin staining kit (UltraSensitiveTM SP kit, Maixin-Bio, Fujian, China) and diaminobenzidine, as previously described[16]. MT and α-SMA staining was quantified using IBAS 2.5 software (Germany) and the data expressed as percentage of positive staining. The average of the score taken from five random fields was used to generate a single per slide.
A colorimetric assay was performed as described[16]. Briefly, lyopholized liver sections (0.5 g) were hydrolyzed for 20 h in 6 mol/L HCl at 100 °C, redissolved in water, and centrifuged to remove any impurities. Samples were incubated for 10 min in 0.05 mol/L chloramine-T (Fisher, Fair Lawn, NJ, USA) at room temperature, followed by 15-min incubation in Ehrlich’s-perchloric acid solution at 65 °C. Sample absorbencies were assessed at 561 nm and resulting values compared to a Hyp standard curve. Each sample was assayed in duplicate. The Hyp content was expressed as micrograms per gram of wet liver.
ALT and TBIL were assessed using our routine laboratory methods. Serum HA and LN were also measured with the kit (Navy Medical Institute, Shanghai, China) by radioimmunoassay.
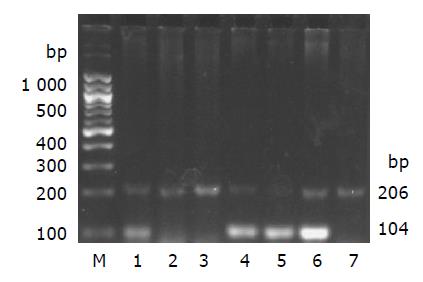
Genomic DNA was prepared from liver tissue of the rats in each group using genomic DNA purification kit (G&T Biotech, MD, USA). The presence or absence of sex determination region on the Y chromosome (sry) gene of liver in recipient female rats was assessed by PCR. The presence of DNA in all tissues was assessed by analysis of the “house-keeping” gene β-actin. Primer sequences for sry gene (forward 5’-catcgaagggttaaagtgcca-3’, reverse 5’-atagtgtgtaggttgttgtcc-3’) were obtained from published sequences[17,18] and amplify a product of 104 bp. β-actin primers (forward 5’-tgttgtccctgtatgcctct-3’, reverse 5’-taatgtcacgcacgatttcc-3’) were designed from GenBank (accession no. J00691) and amplify a product of 206 bp. The PCR conditions were as follows: incubation at 94 °C for 4 min; 35 cycles of incubation at 94 °C for 50 s, 60 °C for 30 s, and 72 °C for 1 min; with a final incubation at 72 °C for 10 min. PCR products were separated using 2% agarose gel electrophoresis, stained with ethidium bromide and imaged by Image Station 200R (Kodak, USA). Positive (male Fischer 344 rat genomic DNA) and negative (female Wistar rat genomic DNA) controls were included in each assay.
Data are expressed as mean±SD. Significant differences were determined by using ANOVA in SPSS 9.0. Results were considered significant when P<0.05.
Figure 1 shows that BM-derived MSCs had differentiated into neural-like cells, adipocyte, and osteoblasts in different conditions (Figures 1A-1C).
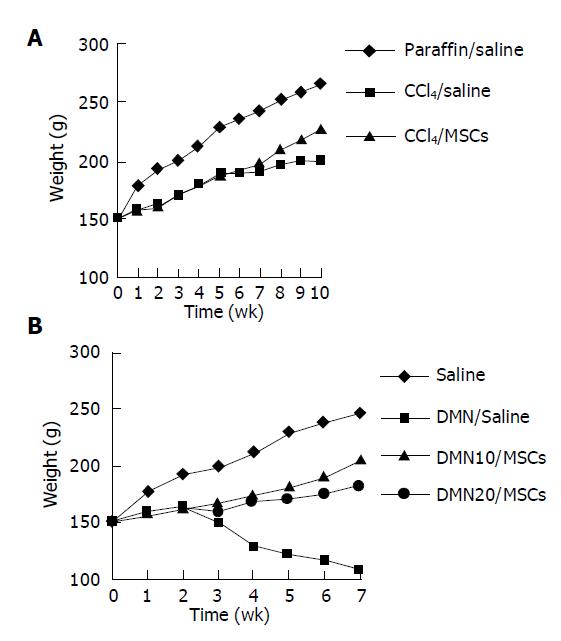
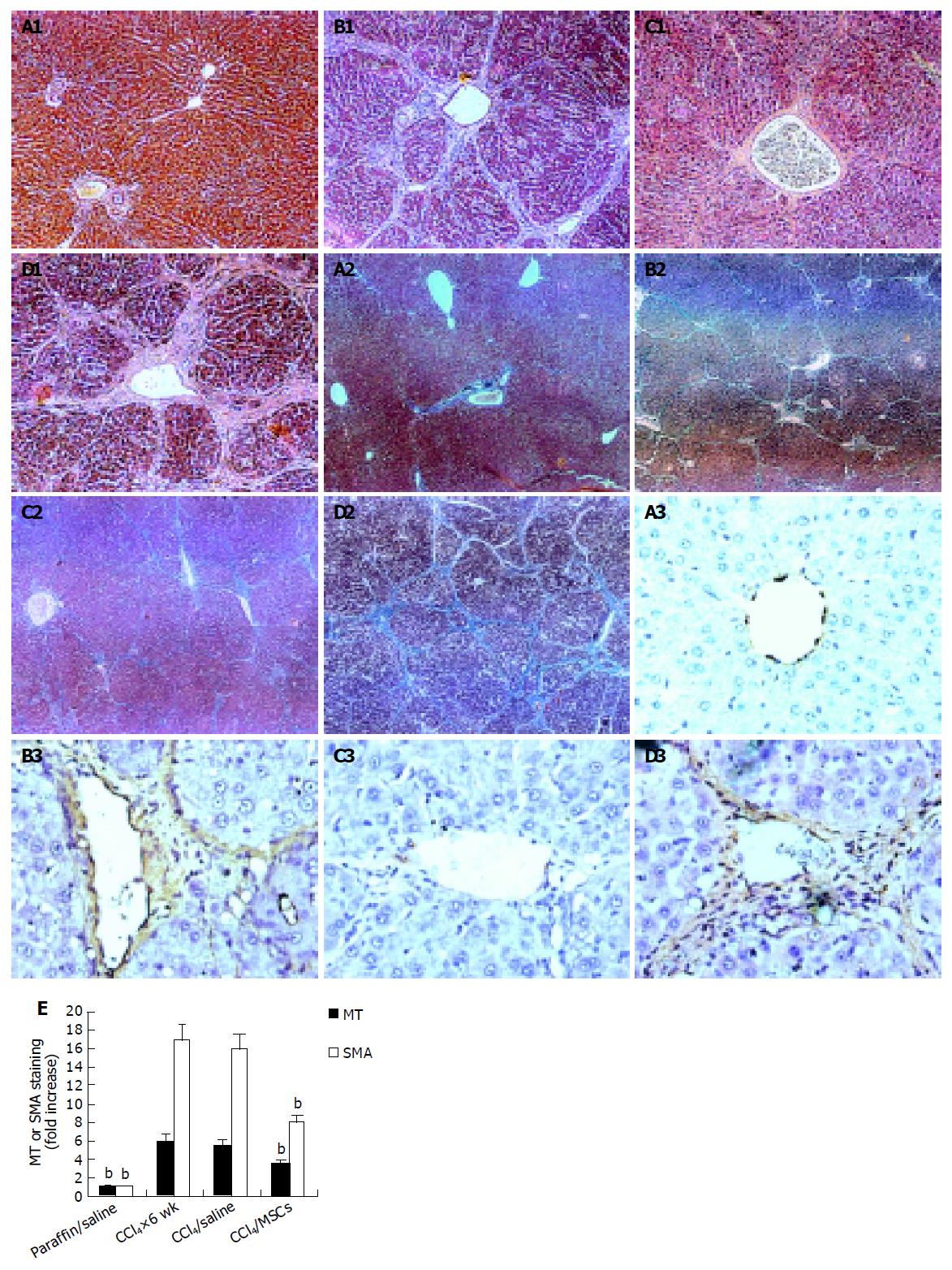
Rats given CCl4 showed slower weight gain than rats with paraffin oil alone (Figure 2A). The average weight of rats with CCl4 for 6 wk was 50-70 g lower than rats with paraffin oil alone. The mortality rates of incidence were about 10% in rats with CCl4 after 6 wk, while the paraffin oil-treated rats were all alive. Up to d 21 of exposure to CCl4, the substantial fibrosis was not observed by histological examination. We found massive rearrangement of the hepatic architecture, including septal fibrosis, extensive bridging, inflammation and extensive fatty changes after 6 wk, while rats with only paraffin oil had normal liver architecture (Figures 3A1 and 3B1). These results were corresponding with previous findings[19].
We also established the other fibrosis model induced with DMN, because there were differences of hepatotoxicity between CCl4 and DMN[20]. The body weight was obviously lower in rats with DMN for 3 wk than that in rats with only saline (Figure 2B). If the body weight fell to less than 100 g, the rat was likely to die soon. The first rat died on d 10 after DMN administration. By d 21, 20% of rats died. Histopathology showed centrilobular congestion and hemorrhagic necrosis liver of rats after 1 wk. Centrilobular necrosis and intense neutrophilic infiltration were observed on d 14. By d 21, collagen fiber deposition could be observed, together with severe centrilobular necrosis and bridging necrosis, with bile duct proliferation and fibrosis surrounding the central veins. These results were corresponding with previous findings[16,21].
To further verify fibrosis, we performed immunohistochemical staining of liver sections for α-SMA, and examined levels of LN, HA, ALT, and TBIL in serum and liver Hyp contents (Figure 3E and Table 1).
On d 42 after CCl4 treatment, rats were infused with MSCs or saline. We found that rats with MSCs had got good morphological and behavioral changes after 7th d, while rats with saline appeared extremely lethargic. Two rats with saline died before the completion of the protocol, while rats with MSCs were all alive. There was a slight lift of weight in CCl4/MSCs compared with CCl4/saline (Figure 2A). We also observed that some rats in CCl4/saline had a large amount of ascites while being killed, which could be a reason for gain of weight while living.
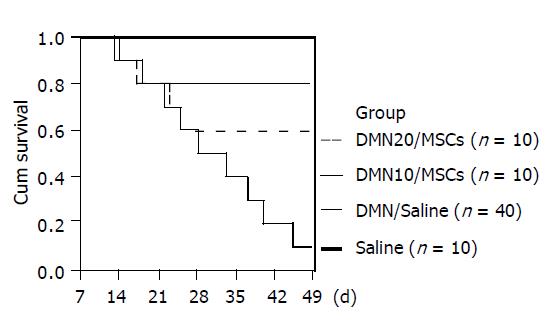
There were dramatic changes of rats with MSCs after 7th d in DMN-induced model (Figure 2B). Rats exhibited grooming, but piloerection, their food and water intake were much increased and their weights increased faster. Rats without MSCs had bad good morphological and behavioral changes. Their weights gain stopped. Their eyes were pale and urines were yellow, and some animals had labored respiration. Thirty-six of forty rats in DMN/saline died of progressive liver fibrosis within 49th d after DMN administration. Life time of rats survived in DMN10/MSCs and DMN20/MSCs was significantly longer than that of rats in DMN/saline (Figure 4). Further, amounts of survival rats in DM10/MSCs were more than those in DMN20/MSCs.
We analyzed liver histology in rats with MSCs in two models by HE staining and MT staining. HE staining showed that architecture of liver got better in CCl4/MSCs (Figure 3C1). Inflammation reduced and pseudolobules were resolved. Collagen accumulation and fatty degeneration were significantly lower in CCl4/MSCs compared with CCl4/saline (Figures 3C1, 3C2, 3D1, and 3D2). Quantification of MT staining demonstrated more than 40% decrease of collagen in liver of rats in CCl4/MSCs compared to that of rats in CCl4/saline (Figure 3E). Meanwhile, the liver histology also got improved in rats with MSCs treatment whether in DMN10/MSCs or in DMN20/MSCs (Figures 5C1 and 5D1). The necrosis of hepatocyte was replaced by the regenerate. The thickened septal fibrosis became thinner or disappeared. Moreover, the degree of fibrosis was decreased in DMN10/MSCs than that in DMN20/MSCs by quantification of MT staining (Figure 5F). It implied that earlier the MSCs were treated, greater was the effect of MSCs on the inhibition of fibrosis.
We examined levels of HA and LN in serum and liver Hyp contents, which are biochemical markers of liver fibrosis. The result showed that the levels of markers significantly decreased in rats with MSCs than that in rats without MSCs in two models (Tables 1 and 2). And reduction of levels of Hyp and HA and LN was more obvious in DMN10/MSCs than that in DMN20/MSCs. These results were consistent with the histology of liver.
| Group | n | HA (mg/L) | LN (mg/L) | TBIL (mg/L) | ALT (U/L) | Hyp (mg/g) |
| Saline | 10 | 142.3±18.9a | 44.5±16.4a | 5.1±1.5a | 32.7±6.8a | 450±110a |
| DMN10 | 10 | 338.7±60.3a | 82.3±23.5a | 8.7±1.8a | 85.3±12.5a | 760±210a |
| DMN20 | 10 | 583.5±59.6a | 114.9±44.2a | 10.8±3.5a | 162.7±22.7a | 1250±450a |
| DMN10/saline | 4 | 981.4±110.5 | 214.2±56.5 | 21.6±5.5 | 212.1±51.4 | 2250±650 |
| DMN10/MSCs | 8 | 395.2±43.1ab | 83.1±43.1ab | 9.1±4.7ab | 67.7±32.9ab | 1010±580ab |
| DMN20/MSCs | 6 | 517.8±82.6a | 98.2±57.2a | 11.1±6.2a | 89.7±38.6a | 1150±750a |
We tested the level of ALT and TBIL in serum, which are primary indexes of liver function. Levels of these two indexes were closed to normal in CCl4/MSCs, but stayed higher in CCl4/saline. We also found that levels were lower in DMN10/MSCs and DMN20/MSCs than that in DMN/saline, although there were few high levels of ALT and TBIL in DMN20/MSCs.
Immunohistochemistry of liver sections for α-SMA expression showed intense staining patterns in CCl4/saline and DMN/saline (Figures 3B3, 3D3, 5A3, 5B3, and 5E3). However, the levels of α-SMA staining in CCl4/MSCs, DMN10/MSCs, and DMN20/MSCs were significantly less (Figures 3C3, 5C3, and 5D3) compared to CCl4/saline and DMN/saline. Quantification of levels of α-SMA staining of liver demonstrated more than 40-50% decrease in rats after MSCs administration in two experimental fibrosis models (Figure 5E). And there were higher levels of α-SMA staining in DMN20/MSCs than that in DMN10/MSCs. This result was consistent with liver histopathology in two models.
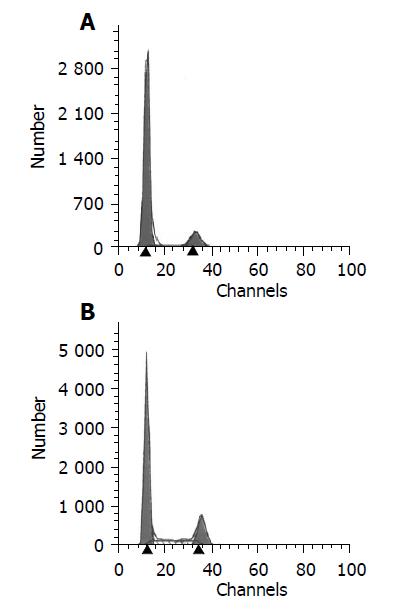
After 4 wk of MSCs treatment, the sry gene was positive by using PCR analysis in the liver of female recipient induced with CCl4. And the similar result was seen in DMN10/MSCs and DMN20/MSCs after 29-39 d of MSCs treatment (Figure 6). These results suggested that BM-derived MSCs were capable of liver engraftment of rats induced with CCl4 or DMN (Figures 7, 8, 9).
In the current study, BM-derived MSCs were isolated and expanded in adult rats and their multiple differentiation potential was also confirmed in vitro. We evaluated the anti-fibrosis effects of infusion of BM-derived MSCs to rats with CCl4- or DMN-induced. The results showed that MSCs treatment could reduce mortality rates of rats and improve the fibrosis index.
CCl4-induced liver fibrosis is a classical experimental fibrosis model. Although this model could undergo spontaneous resolution of fibrosis after stopping CCl4 challenging in some reports[22], we here did not find recovery of liver fibrosis in CCl4/saline within 4 wk of withdrawal of CCl4. In contrast, collagen accumulation and fatty degeneration were significantly lower in CCl4/MSCs compared with CCl4/saline. Further, we also confirmed the anti-fibrosis effects of MSCs by DMN-induced liver fibrosis model. DMN is a hepatotoxin, carcinogen and mutagen, and can cause liver fibrosis. It provides a suitable rapid experimental fibrosis model as like in human liver fibrosis[21,23]. We investigated two groups with MSCs infusion in the different time of fibrosis development in this model. One group DMN10/MSCs was treated with MSCs on the 10th d after DMN administration, in which time it was in early stage of liver fibrogenesis. The other group DMN20/MSCs was infused to MSCs on the 20th d after DMN administration, in which time the fibrosis was forming. Our work demonstrated that infusion of MSCs administration can prolong the life time of rats and improve the liver function and reduce hemorrhagic necrosis collagen accumulation of liver in both groups. Moreover, fibrosis index got more improvement in DMN10/MSCs than that in DMN20/MSCs. These results suggest that infusion of MSCs could not only inhibit the fibrosis formation but also halt the progression of fibrosis in CCl4- or DMN-treated rats.
We[15,24] and others[25] have showed that BM-derived MSCs did not express MHC class II antigens, costimulatory molecules CD80 and CD86. This phenotype is regarded as nonimmunogenic and suggests that MSCs might be effective in inducing tolerance. It was identified that allogeneic or xenogeneic MSCs could persist into hosts after infusion[26]. We here employ BM-derived MSCs from Fischer 344 rats to infuse Wistar rats with liver fibrosis and get a good result. It implies that infusion of allogeneic MSCs is a great value for liver fibrosis in clinical practice.
In the current study the male donor cells was confirmed in the injured liver of female recipients by PCR for the sry gene. However, the mechanism by which MSCs repair the fibrosis is unclear. Fang et al[6], found that although albumin-positive donor-derived cells were present at lower frequency in sections of CCl4-induced liver tissues, infusion of Flk1+ murine MSCs might ameliorate liver fibrosis. Ortiz et al[5], observed that MSCs administration reduced the degree of bleomycin-induced inflammation and collagen deposition within lung tissue, but male donor DNA accounted for 2.21×10-5% of total lung DNA in female recipient mice with MSCs treatment. Kinnaird et al[27], and Silva et al[28], thought that MSCs could secrete a wide array of arteriogenic cytokines and contribute to reducing fibrosis through paracrine mechanisms. These results suggested that MSCs themselves could not functionally rescue the recipients by substituting the damaged cells directly[29]. Although one limitation of the current study is that the quality or quantity of MSCs engraftment in liver was not clear, the fact that MSCs have anti-fibrosis effects in injured liver was clearly proved. We suggest that MSCs may protect against CCl4- or DMN-induced injury by altering the microenvironment of liver at sites of engraftment. In the present study, levels of α-SMA positive staining of liver sections have more than 50% decrease after MSCs administration in two models (Figures 3E and 5F). This is an exciting event because the expression of α-SMA in liver is an indicator of hepatic stellate cells (HSCs) activation, which is recognized as the key players in liver fibrogenesis[1]. For example, MSCs could secrete many cytokine and growth factors, such as hepatic growth factor[30], which shows anti-apoptotic activity in hepatocytes and plays an essential part in the regeneration of the liver[21] and nerve growth factor[9], which can induce apoptosis of cultured HSCs[31]. And recently, we have provided some evidences that in vitro coculture MSCs and HSCs can increase the number of HSCs in G0 phase and reduce the number of HSCs in S phase (unpublished). Thus, MSCs may play a inhibition role in process of HSCs transition from the quiescent state to activated state.
In summary, this data reveals that infusion of BM-derived MSCs can protect against liver fibrosis and the mechanisms of the anti-fibrosis by MSCs will be studied further.
The authors thank Professor Min Xiong and Professor Run-De Hu for help in histopathology, and the staff of Center for Stem Cell Biology and Tissue Engineering, Sun Yat-Sen University.
| 1. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1312] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 2. | Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004;36:2255-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Wen GM, Li HW, Xiao QZ, Cheng ZG, Zhang XM, Li Y, Duan LN, Li SN. Studies on differentiation potential of human bone marrow mesenchymal stem cells into hematopoietic cells in vivo. Zhongguo Bingli Shengli Zazhi. 2003;19:157-162. |
| 5. | Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407-8411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1064] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 6. | Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1147] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 8. | Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1555] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 9. | Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 704] [Article Influence: 29.3] [Reference Citation Analysis (19)] |
| 10. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 664] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 664] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 12. | Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 558] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3924] [Article Influence: 163.5] [Reference Citation Analysis (5)] |
| 14. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 15. | Xiao QZ, Li HW, Wen GM, Liu JB, Zhang XM, Li Y, Li SN. Study on the biological characteristics of rat bone marrow mesenchymal stem cells. Zhongguo Bingli Shengli Zazhi. 2004;3:289-294. |
| 16. | Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Wu GD, Tuan TL, Bowdish ME, Jin YS, Starnes VA, Cramer DV, Barr ML. Evidence for recipient derived fibroblast recruitment and activation during the development of chronic cardiac allograft rejection. Transplantation. 2003;76:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | An J, Beauchemin N, Albanese J, Abney TO, Sullivan AK. Use of a rat cDNA probe specific for the Y chromosome to detect male-derived cells. J Androl. 1997;18:289-293. [PubMed] |
| 19. | Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Yasuda M, Okabe T, Itoh J, Takekoshi S, Hasegawa H, Nagata H, Osamura RY, Watanabe K. Differentiation of necrotic cell death with or without lysosomal activation: application of acute liver injury models induced by carbon tetrachloride (CCL4) and dimethylnitrosamine (DMN). J Histochem Cytochem. 2000;48:1331-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 473] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 840] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Li HW, Wen GM, Xiao QZ, Li HL, Duan LN, Xiang P, Zhang XM, Li SN. Effects of cotransplantation of donor-derived bone marrow mesenchymal stem cells on acute graft versus host disease. Zhongguo Bingli Shengli Zazhi. 2003;5:577-580. |
| 25. | Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1090] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 26. | Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 27. | Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 991] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 28. | Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 571] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 29. | Kanazawa Y, Verma IM. Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11850-11853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Matsuda-Hashii Y, Takai K, Ohta H, Fujisaki H, Tokimasa S, Osugi Y, Ozono K, Matsumoto K, Nakamura T, Hara J. Hepatocyte growth factor plays roles in the induction and autocrine maintenance of bone marrow stromal cell IL-11, SDF-1 alpha, and stem cell factor. Exp Hematol. 2004;32:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Trim N, Morgan S, Evans M, Issa R, Fine D, Afford S, Wilkins B, Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000;156:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK