Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3217
Revised: December 29, 2003
Accepted: January 8, 2004
Published online: June 7, 2005
AIM: To study the effect of IGF-1/IGF-1R and gastrin/CCK-BR on carcinogenesis and development of human gastric carcinoma and to explore its mechanism and provide a credible theoretical foundation for early diagnosis and molecular therapy of gastric carcinoma.
METHODS: mRNA expression levels of IGF-1/IGF-1R and gastrin/CCK-BR were assessed by RT-PCR method in gastric cancer tissues, adjacent mucosa, and tumor-free tissues from 56 patients with gastric carcinoma and normal gastric mucosae from 56 healthy controls. Tissue specimens were obtained by biopsy and confirmed by histological evaluation.
RESULTS: The mRNA levels of IGF-1/IGF-1R were increased in gastric cancer tissues compared with normal tissues from healthy controls and successively increased in tumor-free tissues, adjacent mucosa, and gastric cancer tissues. The mRNA levels of gastrin/CCK-BR were increased in gastric cancer tissues compared with normal tissues from healthy controls. There was a significant difference between gastric cancer tissues and adjacent mucosa and tumor-free tissues, but the mRNA levels of gastrin were not significantly increased in adjacent mucosa and gastric cancer tissues compared with tumor-free tissues. The mRNA levels of CCK-BR were increased in gastric cancer tissues and adjacent mucosa compared with tumor-free tissues, but not significantly increased in adjacent mucosa and gastric cancer tissues compared with gastric cancer tissues.
CONCLUSION: Overexpression of IGF-1/IGF-1R and gastrin/CCK-BR promotes the disorderly proliferation of gastric mucosa epithelia and it is of great significance in the carcinogenesis and development of gastric carcinoma.
- Citation: Zhao MD, Hu XM, Sun DJ, Zhang Q, Zhang YH, Meng W. Expression of some tumor associated factors in human carcinogenesis and development of gastric carcinoma. World J Gastroenterol 2005; 11(21): 3217-3221
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3217
Gastric cancer is one of the most frequent neoplasms and a leading cause of death worldwide. It is still the focus of study to explore the mechanism of gastric carcinoma. With the development in molecular biology technology, research on the mechanism of gastric carcinoma has gone deep into the molecular levels. Carcinogenesis is a complicate process, which includes many steps, many gene abnormities may be brought on this process. Some researchers considered that the effect of protooncogenes, the inactivation of many antioncogenes, the regulation of many growth factors and their receptors, the accumulation of gene mutations and the imperfection of DNA rehabilitation system might play important roles in the carcinogenesis and development of gastric carcinoma. There is evidence that the upregulation of certain growth factors could play an important role in the promotion and development of gastric cancer[1,2].
The insulin-like growth factor system is widely involved in human carcinogenesis[3]. The type 1 insulin-like growth factor receptor (IGF-1R) is a transmembrane protein tyrosine kinase, which mediates the biological effects of IGF-1[4], and plays an important role in both normal and abnormal growth, particularly in anchorage independent growth. Some investigators also provided evidence that insulin-like growth factor-1 (IGF-1) and its receptor (IGF-1R) emerged as key regulators of mitogenesis and tumorigenicity. It has been well established that a functional IGF-1R is required for cell growth and plays a crucial role in survival of various transformed cells in vitro and in vivo[5]. When IGF-1 combines with its receptor (IGF-1R), it has an important effect on human metabolism of proteins and carbohydrates, and stimulates cell proliferation, differentiation and apoptosis. These changes of cell activity are the characteristics of carcinoma cells. Durrant et al[6], have reported that IGF-1 could facilitate the growth of three cell strains of gastric carcinoma in vitro. Peters et al[7], had found that IGF-1 and its membrane receptors played a critical role in the carcinogenesis of several tumors, such as colorectal cancer. More recently, numerous studies have demonstrated that IGF-1R can play a critical role in regulation of carcinoma metastasis and invasion, and that overexpression of IGF-1R is an important component controlling the proliferation of cervical carcinoma cells[8-10]. However, the mechanism underlying gastric cancer and the exact role of IGF-1/IGF-R in human gastric carcinogenesis and development remain poorly understood.
Gastrin is one of the most extensively researched gastrointestinal hormones and is of great significance in sustaining normal physiological functions of gastrointestinal tract. Research has demonstrated that gastrin can accelerate the growth of cultured gastric carcinoma cells[11] and transplanted human gastric carcinoma in naked mice[12] can increase the synthesis of DNA and proteins. There is evidence that expression of gastrin mRNA is increased in gastric cancer and release of gastrin is increased in plasma and gastric lumen of patients with gastric cancer[13]. So it may be a link between gastrin and gastric cancer. The role of gastrin in the carcinogenesis and development of gastric cancer needs to be explored deeply.
Cholecystokinin B/gastrin receptors (CCK-BR) can stimulate the secretion of gastric acid and cell proliferation[14,15]. The expression of CCK-BR has been widely reported in colorectal cancer[16,17]. However, the role of gastrin/CCK-BR in the carcinogenesis and development of human gastric carcinoma is still largely unknown.
The aim of the present study was to evaluate the effect of IGF-1/IGF-1R and gastrin/CCK-BR on the carcinogenesis and development of human gastric carcinoma, and to explore its mechanism and provide a credible theoretical foundation for early diagnosis and molecular therapy of gastric carcinoma.
Fresh tissue specimens of gastric cancer tissues, adjacent mucosa and tumor-free tissues were obtained from 56 patients with gastric carcinoma. Specimens of normal gastric mucosae were from 56 healthy controls. The specimens were immediately frozen in liquid nitrogen and stored at -80 °C. The diagnosis of specimens was based on the pathological features of tumors and confirmed by pathological histological evaluation.
The extraction of total RNA from tissues was carried out as previously described[1]. Briefly, total RNA was extracted using a guanidium isothiocyanate/phenol chloroform single step extraction kit from Stratagene (Promega, USA) based on the method previously described[18]. Following precipitation, RNA was resuspended in RNase-free water and its concentration was estimated by absorbance at 260 nm wavelength. Furthermore, the quality of each RNA sample was determined by agarose-formaldehyde electrophoresis. RNA samples were stored at -80 °C until analysis. The nucleotide sequences of primers are shown in Table 1.
| Primer | Sequence | PCR product (bp) |
| IGF-1 | 5-CAACAAGCCCACAGGGTATGGC-3’ | |
| 3-GGAAACGAGACGTGCTCAATGGACA-5’ | 312 | |
| IGF-1R | 5-ATGCTGTTTGAACTGATGCGCA-3’ | |
| 3-GCCCCCGGCGTTCTTGCTCGCC-5’ | 354 | |
| Gastrin | 5-GTCTATGTGCTGATCTTTGCACTG-3’ | |
| 3-CTTGGTTCGAAGTCTCGGATCGGT-5’ | 323 | |
| CCK-BR | 5-GTGGCCTACGGGCTTATCTCTCGCGAGCTCTACTTA-3’ | |
| 3-GACACAACCAACGGTCAAATATCACGGTTGTGCA-5’ | 357 | |
| β-actin | 5-GGCGGCACCACCATGTACCCT-3’ | |
| 3-TCATACTGCTCAGGCCGGGGA-5’ | 202 |
The primers were synthesized and purified by Cell Biology Institute, Chinese Academy of Sciences.
RT-PCR analysis was done routinely according to instruction of reagent box, using an access RT-PCR system (American Promega Company). β-actin was used as an inner-control and the template dosage of cDNA was standardized. The reaction conditions of RT-PCR were as follows.
IGF-1: denaturation at 94 °C for 30 s; annealing at 57 °C for 30 s; Extension at 72 °C for 2 min; 35 circles at 72 °C for 7 min and stored at 4 °C.
IGF-1R: denaturation at 94 °C for 30 s; annealing at 54 °C for 30 s; extension at 72 °C for 1 min; 35 circles at 72 °C for 7 min and stored at 4 °C.
Gastrin: denaturation at 94 °C for 30 s; annealing at 59 °C for 30 s; extension at 68 °C for 1 min; 35 circles at 72 °C for 7 min and stored at 4 °C.
CCK-BR: denaturation at 94 °C for 30 s; annealing at 55 °C for 30 s; extension at 72 °C for 1 min; 35 circles at 72 °C for 7 min and stored at 4 °C.
Ten microliters of the PCR products of IGF-1/IGF-1R and gastrin/CCK-BR and β-actin was detected by electrophoresis on an 1.8% agarose gel. The results were scanned and photographed by an automatic gel image analysis system (Biotechnology Limited Corporation, Coldspring Harbor, USA). The result was expressed as investigated PCR-product of target factor/β-actin mRNA ratio.
Data were presented as mean±SE. Statistical analysis was performed using Student’s t test. P<0.05 was considered statistically significant.
The extracted RNA showed two pieces of bands 18 and 28 s in the agarose gel electrophoresis. After RNA was detected by an ultraviolet spectrophotometer, the A260/A280 ratio surpassed 1.7.
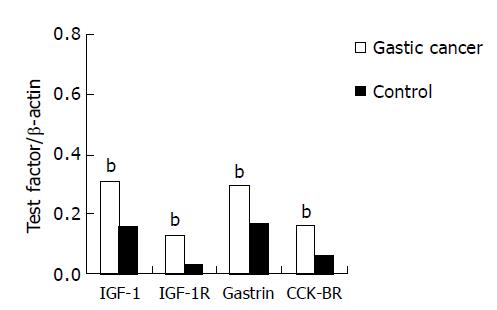
The mRNA expression levels of IGF-1/IGF-1R and gastrin/CCK-BR in gastric cancer tissues were all increased significantly compared with normal mucosa from controls (P<0.01). The result is shown in Table 2 and Figure 1.
The mRNA expression levels of gastrin were successively increased in tumor-free tissues, adjacent mucosa and gastric cancer tissues. There was a significant difference between gastric cancer tissues and adjacent mucosa and tumor-free tissues (P<0.05), but there was no significant difference between adjacent mucosa and tumor-free tissues (P>0.05). The mRNA expression levels of CCK-BR were also successively increased in tumor-free tissues, adjacent mucosa and gastric cancer tissues. There was significant difference in gastric cancer tissues and adjacent mucosa compared with tumor-free tissues (P<0.05), but there was no significant difference between gastric cancer tissues and adjacent mucosa (P>0.05, Table 3).
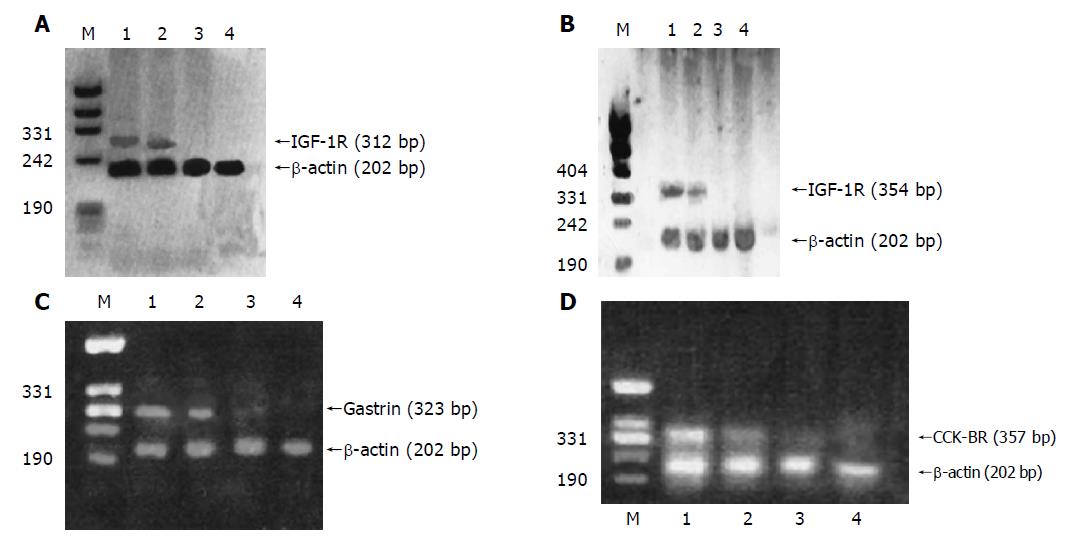
The RT-PCR amplified products of IGF-1, IGF-1R, gastrin, CCK-BR and β-actin in three kinds of tissues from patients with gastric carcinoma were detected by electrophoresis on an 1.8% agarose gel. The results are shown in Figure 2.
IGF-1 is a member of IGF family. It is a single chain polypeptide (MW 7.65 ku) containing 70 amino acids and 6 exons[19]. Its biological responses are transmitted by IGF-1R. The results of some studies showed that IGF-1 could stimulate numerous types of tumor cells proliferating through binding to IGF-1R[20]. IGF-1R is a glycoprotein tetramer located in cell membranes and involves 2 α subunits (MW 100-135 ku) with binding sites for ligands and 2 β subunits (MW 90-95 ku) containing transmembrane PTK active areas[21]. IGF-1 not only has an important effect on in vivo metabolism of proteins and carbohydrates through binding to the cell-surface IGF-1 receptors (IGF-1R) but also regulates cell activity including cell proliferation, differentiation and apoptosis and these changes are the characteristics of carcinoma cells. Experiments showed that IGF-1 could stimulate cell DNA synthesis, upregulate expression of cyclin D1 and motivate cell mitosis from phase G1 to phase S in vitro. At the same time, it could upregulate Bcl-2 and Bcl-xL levels and downregulate Bax to inhibit cell apoptosis and stimulate cell proliferation[22]. The function of IGF-1R is similar to IGF-1 but it could affect cell conversion. Other studies showed that IGF-1R was overexpressed and the activity of IGF-1R was increased in breast cancer[23,24]. Up to now, only a few studies on IGF-1/IGF-1R mRNA expression in gastric carcinoma tissue are available. In this study, we measured the expression of IGF-1 and its receptors at mRNA level in order to analyze the possible correlation between the activity of these genes and cell proliferation in human gastric tumors. The results showed that the mRNA expression levels of IGF-1/IGF-1R were higher in cancer tissues than in normal gastric tissues. We consider that IGF-1 overexpression could result in overexpression of IGF-1R. The overexpressed IGF-1R was bound to IGF-1 to stimulate gastric excess mucosa cell proliferation, abnormal conversion and apoptosis, resulting in gastric mucosa canceration. Our results clearly demonstrated the important role of IGF-1/IGF-1R in the pathogenesis of gastric cancer, including that the overexpression of IGF-1/IGF-1R plays an important role in the carcinogenesis and development of gastric carcinoma. In addition, it supports the hypothesis that autocrine/paracrine stimulation of cell growth by IGF-1, which may be an important mechanism in the pathogenesis of invasive and metastatic gastric cancer. The simultaneous targeting of growth factor receptors in IGF-1-producing and -dependent cancer cells might be a therapeutic strategy.
In an additional attempt to evaluate the mechanism of IGF-1/IGF-1R potential contributions to gastric cancer growth or progression, we detected the expression level of tumor-free tissues, adjacent mucosa and gastric cancer tissues. The result showed that the mRNA expression level was successively increased in tumor-free tissues, adjacent mucosa to gastric cancer tissues. These findings suggest that there must be a molecular change during the transition from normal to malignant gastric cells that allows IGF-1 or/and IGF-1R to play a stimulatory role. One possibility was the activation or inactivation of a regulatory ‘switch’ molecule that alters cellular physiology in a way that favors IGF-1/IGF-1R carcinogenesis role. But we do not know at which step the overexpression of IGF-1/IGF-1R occurs and which factors can regulate it. The mRNA expression level of IGF-1/IGF-1R was obviously higher in gastric cancer tissues and adjacent mucosa than in normal tissue. So we deduced that the overexpression of IGF-1/IGF-1R might be used as an indicator for early diagnosis of gastric carcinoma.
Gastrin is a member of gastrin/CCK family. It contains three exons. Aside from the function of regulating the secretion of gastric acid, we have found that it plays an important role in the carcinogenesis. CCK-BR is a gastrin receptor[25] and belongs to the G-protein correlative receptor superfamily. It could activate a sequence of enzymic reaction, and call Ca2+ inside cells, and finally activate protein kinase C[26]. Gastrin/CCK-BR could also promote the growth of gastric carcinoma[27,28], and proliferate gastric carcinoma cells[29].
The present study found that mRNA of gastrin/CCK-BR was expressed both in gastric carcinoma tissues and in normal controls, but it was increased in gastric carcinoma tissues compared with controls (P<0.01). This finding is consistent with previous reports by Konturek et al[30]. It is supposed that a self-secretive circle of gastrin-CCK-BR may exist in gastric mucosa, which regulates the normal secretion of gastric acid and proliferation of gastric mucosa cells. In gastric carcinoma, overexpression of gastrin stimulates the expression of CCK-BR. After combined with gastrin/CCK-BR, proliferation of cells is gradually strengthened, then goes into a disorderly state, and the synthesis of DNA and proteins is increased abnormally, at last the formation of carcinoma is accelerated. Therefore, it is preliminarily considered that overexpression of gastrin/CCK-BR takes part in the carcinogenesis and development of gastric carcinoma. However, its mechanism should be further studied.
With the research of gastric carcinoma deepended, it is the focus to find out pathological changes in the prophase of carcinoma and signs of early pathological changes. However, the highly specific and sensitive method has not been found until now. The present study detected the mRNA expression levels of gastrin/CCK-BR in gastric cancer tissues, adjacent mucosa and tumor-free tissues from patients with gastric carcinoma. The results showed that the levels were increased successively in tumor-free tissues, adjacent mucosa and gastric cancer tissues. Moreover, the mRNA expression of gastrin was increased in adjacent mucosa tissues compared with tumor-free tissues (P>0.05). After analyzing the above results, we believe that gastrin and CCK-BR both take part in the carcinogenesis of gastric carcinoma, but the increase of CCK-BR expression may appear earlier than gastrin expression. As for the reasons, it could be that many factors influence the carcinogenesis of gastric carcinoma and the expression of CCK-BR is also regulated by other members of gastrin/CCK-BR family, such as CCK, so the change of CCK-BR expression is a result of very complicated mutual regulation between receptors and ligands. Early changes in mRNA expression of CCK-BR may be of great significance in estimating the early carcinogenesis of gastric carcinoma.
| 1. | Konturek PC, Konturek SJ, Sulekova Z, Meixner H, Bielanski W, Starzynska T, Karczewska E, Marlicz K, Stachura J, Hahn EG. Expression of hepatocyte growth factor, transforming growth factor alpha, apoptosis related proteins Bax and Bcl-2, and gastrin in human gastric cancer. Aliment Pharmacol Ther. 2001;15:989-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Han SU, Lee JH, Kim WH, Cho YK, Kim MW. Significant correlation between serum level of hepatocyte growth factor and progression of gastric carcinoma. World J Surg. 1999;23:1176-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Torrisi R, Mezzetti M, Johansson H, Barreca A, Pigatto F, Robertson C, Decensi A. Time course of fenretinide-induced modulation of circulating insulin-like growth factor (IGF)-i, IGF-II and IGFBP-3 in a bladder cancer chemoprevention trial. Int J Cancer. 2000;87:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Blakesley VA, Stannard BS, Kalebic T, Helman LJ, LeRoith D. Role of the IGF-I receptor in mutagenesis and tumor promotion. J Endocrinol. 1997;152:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Durrant LG, Watson SA, Hall A, Morris DL. Co-stimulation of gastrointestinal tumour cell growth by gastrin, transforming growth factor alpha and insulin like growth factor-I. Br J Cancer. 1991;63:67-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Peters G, Gongoll S, Langner C, Mengel M, Piso P, Klempnauer J, Rüschoff J, Kreipe H, von Wasielewski R. IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and predictive markers in colorectal-cancer. Virchows Arch. 2003;443:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Long L, Rubin R, Brodt P. Enhanced invasion and liver colonization by lung carcinoma cells overexpressing the type 1 insulin-like growth factor receptor. Exp Cell Res. 1998;238:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Steller MA, Delgado CH, Bartels CJ, Woodworth CD, Zou Z. Overexpression of the insulin-like growth factor-1 receptor and autocrine stimulation in human cervical cancer cells. Cancer Res. 1996;56:1761-1765. [PubMed] |
| 10. | Long L, Rubin R, Baserga R, Brodt P. Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res. 1995;55:1006-1009. [PubMed] |
| 11. | Ishizuka J, Martinez J, Townsend CM, Thompson JC. The effect of gastrin on growth of human stomach cancer cells. Ann Surg. 1992;215:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Sumiyoshi H, Yasui W, Ochiai A, Tahara E. Effects of gastrin on tumor growth and cyclic nucleotide metabolism in xenotransplantable human gastric and colonic carcinomas in nude mice. Cancer Res. 1984;44:4276-4280. [PubMed] |
| 13. | Konturek PC, Konturek SJ, Bielanski W, Karczewska E, Pierzchalski P, Duda A, Starzynska T, Marlicz K, Popiela T, Hartwich A. Role of gastrin in gastric cancerogenesis in Helicobacter pylori infected humans. J Physiol Pharmacol. 1999;50:857-873. [PubMed] |
| 14. | Bordi C, D'Adda T, Azzoni C, Pilato FP, Caruana P. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol. 1995;19 Suppl 1:S8-S19. [PubMed] |
| 15. | Hellmich MR, Rui XL, Hellmich HL, Fleming RY, Evers BM, Townsend CM. Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J Biol Chem. 2000;275:32122-32128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Upp JR, Singh P, Townsend CM, Thompson JC. Clinical significance of gastrin receptors in human colon cancers. Cancer Res. 1989;49:488-492. [PubMed] |
| 17. | Smith JP, Stock EA, Wotring MG, McLaughlin PJ, Zagon IS. Characterization of the CCK-B/gastrin-like receptor in human colon cancer. Am J Physiol. 1996;271:R797-R805. [PubMed] |
| 18. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39210] [Article Influence: 1005.4] [Reference Citation Analysis (0)] |
| 19. | Hoyt EC, Hepler JE, Van Wyk JJ, Lund PK. Structural characterization of exon 6 of the rat IGF-I gene. DNA Cell Biol. 1992;11:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Pavelić K, Kolak T, Kapitanović S, Radosević S, Spaventi S, Kruslin B, Pavelić J. Gastric cancer: the role of insulin-like growth factor 2 (IGF 2) and its receptors (IGF 1R and M6-P/IGF 2R). J Pathol. 2003;201:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Czech MP. Signal transmission by the insulin-like growth factors. Cell. 1989;59:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 279] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Párrizas M, LeRoith D. Insulin-like growth factor-1 inhibition of apoptosis is associated with increased expression of the bcl-xL gene product. Endocrinology. 1997;138:1355-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Surmacz E, Guvakova MA, Nolan MK, Nicosia RF, Sciacca L. Type I insulin-like growth factor receptor function in breast cancer. Breast Cancer Res Treat. 1998;47:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Resnik JL, Reichart DB, Huey K, Webster NJ, Seely BL. Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res. 1998;58:1159-1164. [PubMed] |
| 25. | Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski LF, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci USA. 1992;89:3605-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 333] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 248] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Kaise M, Muraoka A, Seva C, Takeda H, Dickinson CJ, Yamada T. Glycine-extended progastrin processing intermediates induce H+,K(+)-ATPase alpha-subunit gene expression through a novel receptor. J Biol Chem. 1995;270:11155-11160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Watson S, Durrant L, Morris D. Gastrin: growth enhancing effects on human gastric and colonic tumour cells. Br J Cancer. 1989;59:554-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Eden PA, Taylor JE. Somatostatin receptor subtype gene expression in human and rodent tumors. Life Sci. 1993;53:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Konturek PC, Kania J, Kukharsky V, Ocker S, Hahn EG, Konturek SJ. Influence of gastrin on the expression of cyclooxygenase-2, hepatocyte growth factor and apoptosis-related proteins in gastric epithelial cells. J Physiol Pharmacol. 2003;54:17-32. [PubMed] |