Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2662
Revised: March 4, 2004
Accepted: June 7, 2004
Published online: May 7, 2005
AIM: To investigate the changing patterns of glycogen and enzyme histochemical activities in rat liver graft under a different warm ischemia time (WIT) and to predict the tolerant time limitation of the liver graft to warm ischemia injury.
METHODS: The rats were randomized into five groups, WIT was 0, 15, 30, 45, 60 min, respectively, and histochemical staining of liver graft specimens was observed. The recovery changes of glycogen and enzyme histochemistry activities were measured respectively 6 and 24 h following liver graft implantation.
RESULTS: The activities of succinic dehydrogenase, cytochrome oxidase, apyrase (Mg++-ATPase) and content of glycogen were decreased gradually after different WIT in a time-dependent manner. The changes were significant when WIT was over 30 min.
CONCLUSION: Hepatic injury is reversible within 30 min of warm ischemia injury. Glycogen and enzyme histochemistry activities of liver grafts and their recovery potency after reperfusion may serve as criteria to evaluate the quality of liver grafts.
- Citation: He XS, Ma Y, Wu LW, Wu JL, Hu RD, Chen GH, Huang JF. Dynamical changing patterns of glycogen and enzyme histochemical activities in rat liver graft undergoing warm ischemia injury. World J Gastroenterol 2005; 11(17): 2662-2665
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2662.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2662
The shortage of donor livers is a critical limiting factor for liver transplantation in treatment of end-stage liver diseases. Organs from non-heart-beating donors (NHBD) seem to be an option to alleviate this problem effectively. However, the main obstacle to the use of livers from NHBD is warm ischemia to the liver-related cardiac arrest[1,2]. Moreover, in liver transplantation, the allograft sustains inevitable cold ischemia in addition to rewarming injury during liver reperfusion[3,4]. Therefore, warm ischemia-reperfusion injury of liver grafts has become a hot topic with theoretical and clinical significance, and it has drawn more and more attention[3,4]. In this study, we performed a continuous observation of the chemical activities of hepatic glycogens and enzymes in rats under a different warm ischemia time (WIT) by histochemical and cytochemical techniques to provide basic data for further research in similar clinical situations.
Adult healthy male Sprague-Dawley rats, weighing 250-300 g, supplied by Experimental Animal Center in Sun Yat-Sen University, were used for the models. Breeding conditions were in coincidence with specific pathogen-free animal standards. Mean weight of recipient rats was a little heavier than that of donor rats.
A midline laparotomy was performed in supine position after an ether aspiration anesthesia, 0.2 mL of heparin sodium solution (1250 u heparin sodium) was injected via dorsum of penis vein to heparinize the donor liver. Thereafter, we sheared diaphragm, clamped the basilar part of the heart and blocked the thoracic aorta. Thus, a donor liver warm ischemia model was established.
After the predicted warm ischemia duration in each group, 20 mL of 0-4 °C lactic acid Ringer’s solution (50 u/mL heparin sodium) was infused into the abdominal aorta via a catheter. The liver graft turned fulvous when filling solution flowed out via the sheared right atria. All of the liver ligaments were dissected. The pyloric vein was ligated proximal to the portal vein after the hepatic proper artery and portal vein were freed. The infra-hepatic inferior vena cava was isolated and the right suprarenal vein and right renal vein were cut. The supra-hepatic inferior vena cava was cut in the position close to the diaphragm annulus, the hepatic artery was ligated and cut, the portal vein was cut in the confluence of portal vein and splenic vein, and the infra-hepatic inferior vena cava was cut over the left renal vein. Specimens of donor livers were preserved in the 4 °C lactic acid Ringer’s solution. Anesthesia, position and incision were all the same in donor and recipient operations. We modified the angioanastomotic technique[6] on the basis of biculf technique suggested by Kamada and Calne[7] and Sun et al[8]. Cold ischemia time was 50±3.5 min, and hepatic period was 20±2.5 min.
One hundred and fifty rats were randomly divided into five groups, WIT was 0, 15, 30, 45, 60 min respectively. Six of thirty rats in each group were killed for observation of hepatic glycogen and enzyme chemical activities in the warm ischemia stage. OLT was performed for the rest 24 rats in each group, 12 as donors and 12 as recipients. Six of twelve recipient rats were killed for observation in the reperfusion stage 6 and 24 h after transplantation respectively.
Hepatic glycogens were stained by PAS reaction after fresh specimens were fixed in Gendre’s solution for 6 h. Techniques of tetrazolium blue, PPDA, magnesium activation were adopted to observe the activities of succinic dehydrogenase (SDH), cytochrome oxidase (CO) and ATPase respectively on 5-6 μm cryo-sections. The spectrum and distribution for dying were observed under a microscope in a semi-quantitative way and the results were analyzed via 30 random fields with a higher amplification. Sections from the same specimen should be placed on the same sheet glass for auto-comparison and avoidance of interference in the staining process.
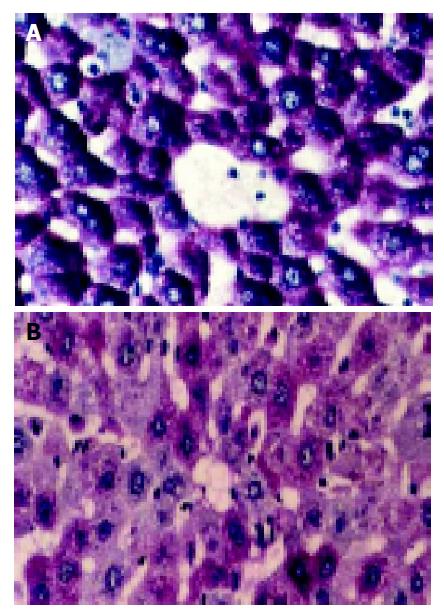
Glycogen staining (PAS reaction) PAS reaction was strongly positive (++++) in normal hepatic cells with abundance of productive glycogen storage. Purplish red particles were distributed in the cytoplasm with “polarity” (Figure 1A). These particles began to decrease once WIT was over 30 min, especially in the lobule center area. Forty-five minutes later, the change was obvious, which had a tendency related with WIT in a time-dependent manner (Figure 1B). Some particles even vanished in 60-min group.
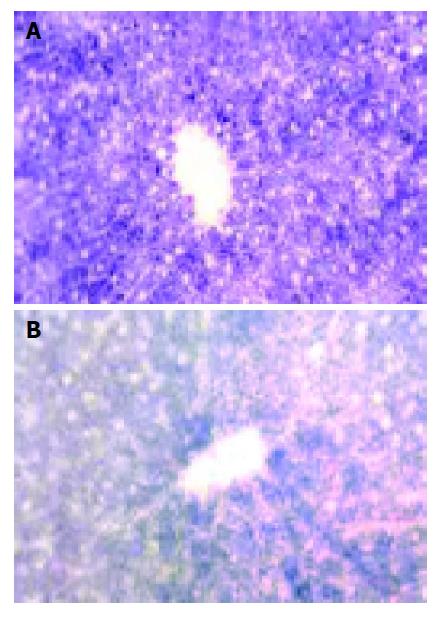
Succinic dehydrogenase (SDH) Deep-blue particles representing the SDH activity located mainly in hepatic parenchymal cytoplasm, hepatocytes around the portal vein had more abundant and deeper particles than those around the center vein (Figure 2A). SDH activities began to show a plaque-like decrease 45 min after warm ischemia, while this change was slight in groups, with their WIT was less than 30 min (Figure 2B).
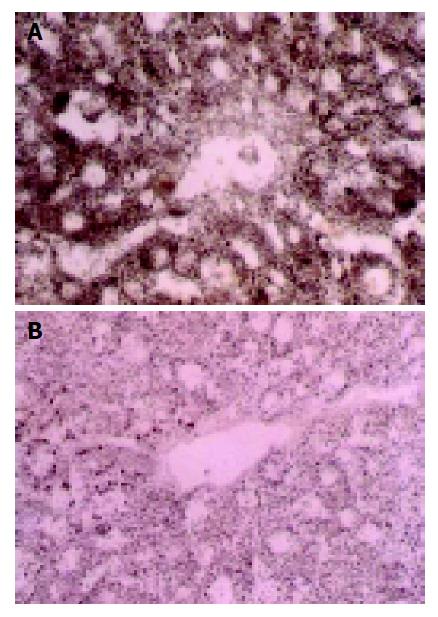
Cytochrome oxidase (CO) Brown particles deposited in areas with CO activity, which only presented in mitochondria, were especially abundant in those of the lobule center area (Figure 3A). CO activity changed slightly in groups with their WIT less than 30 min, but significantly in groups with their WIT over 30 min (Figure 3B).
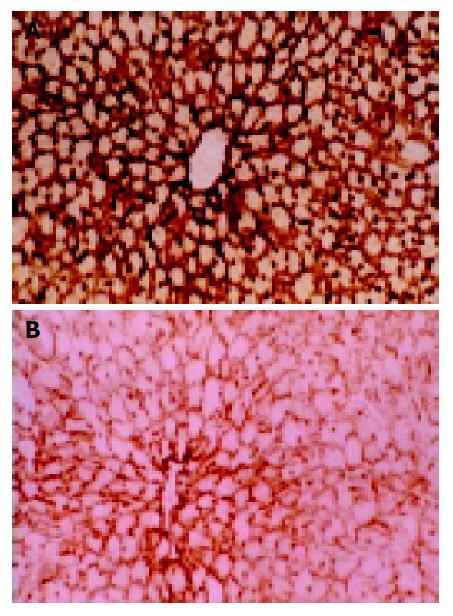
ATPase Brown-dark staining was seen in positions with normal ATPase activity, which only presented in hepatocytic membranes and cholangioles (Figure 4A). Enzyme activity decreased slightly 45 min after warm ischemia, while in 60-min group, this change could involve a sheet or foci-like area ranging from several hepatic lobules to several cells limited in one lobule. Cells with normal enzyme activities could be seen in the area around the portal vein and the center vein; therefore, the observation was often characterized by the interlock of cells with different enzyme activities (Figure 4B).
Changes of hepatic glycogen and enzyme histochemical activities were related to WIT significantly in a time-dependent manner, as shown in Table 1.
| Observation indexes | 0-min group | WIT 15-, 30-min groups | WIT 45-, 60-min groups | ||||||
| Contrast | Reperfusion 6 h | Reperfusion 24 h | After ischemia | Reperfusion 6 h | Reperfusion 24 h | After ischemia | Reperfusion 6 h | Reperfusion 24 h | |
| PAS | ++++ | ++-+++ | +++-++++ | ++-+++ | +-++ | +++ | +-± | ±-- | + |
| SDH | ++++ | ++ | +++ | +++-++++ | +-++ | ++-+++ | + | ± | +-± |
| CO | ++++ | ++ | +++ | ++++ | +-++ | ++-+++ | +-++ | +-± | +-± |
| ATPase | ++++ | +++ | +++ | +++ | ++ | ++-+++ | +-± | ± | + |
In groups with WIT less than 30 min, PAS reaction and enzyme histochemical activities showed a further decrease 6 h after transplantation. Twenty-four hours after reperfusion, hepatic glycogen resumed to normal level and SDH, CO and ATPase activities also recovered gradually to normal level. While in groups with WIT over 30 min, glycogen and enzyme histochemical activities decreased gradually 6 h after implantation. In addition, the recovery potency was slight 24 h after implantation.
The disparity between the increasing demand for liver donors and the limited supply of donor organs has led to a reconsideration of the use of marginal pools such as NHBD[1,2,10]. NHBD programs have been used successfully in kidney transplantation, and are able to increase donor pool of kidneys by 40%. In the field of liver transplantation, the use of livers from NHBD showed a less favorable clinical result, because of the limited hepatic tolerance against warm ischemic damage compared with kidneys[11,12]. Although several clinical and experimental studies have shown that liver can compensate for 60 min of WIT during liver resection, in transplantation settings there were few studies about the limit of the period of warm ischemia before transplantation[13].
In the present study, a dynamic observation of rat liver graft specimens in a different WIT was made by histochemical and cytochemical methods. We found that hepatic SDH, CO and ATPase activities and glycogen decreased gradually with the elongation of WIT in a time-dependent manner. The study showed that changes of SDH, CO and ATPase activities were slight when WIT was within 30 min, while in 45- and 60-min groups, a remarkable decrease of enzymes was noted. We also found progressive cytoplasm rarefaction, hepatocyte edema and vacuolation without obvious cell necrosis under a microscope with routine HE staining in a different WIT[14,15]. Dynamic observation also enabled us to combine the morphology and metabolic changes to reflect the degree of hepatic cell injury more sensitively and accurately.
SDH located in the inner mitochondrial membrane is an important enzyme in cellular oxidation and oxidative phosphorylation of tricarboxylic acid cycle (TAC), as a marker enzyme of mitochondria. Its activity reflects the function of TAC. CO as the last enzyme link in mitochondrion oxidative phosphorylation chain also is regarded as the key enzyme in mitochondria. ATPase is a hydrolase, it could hydrolyze ATP into ADP with release of energy[16]. We can conclude from our study that, after the blockage of hepatic blood supply, hepatocyte mitochondria undergo dysfunction secondary to ischemia and anoxia, mitochondrial oxidative phosphorylation is inherited with gradual decrease of SDH, CO and ATPase activities. In the initial stage of hepatic ischemia, hepatocytes underwent a rapid glycogenolysis and anoxic glycolysis to compensate for the energy insufficiency, thus cellular and subcellular structure and function could be maintained[17]. With the elongation of WIT, glycogen and ATP decreased gradually even exhausted, which could lead to irreversible injury in ischemia cells.
From our study, we can conclude that donor livers from NHBD undergo ischemia injury both in the warm ischemia period and in the reperfusion period. Hepatic glycogen and enzyme activities are positively related to WIT in a time-dependent manner during the reperfusion period. In 15- and 30-min WIT groups, PAS reaction and enzyme histochemical activities showed a recovering potency. While in 45- and 60-min groups, the liver graft underwent an irreversible injury; therefore, no evident recovery potency was seen 24 h after implantation[18]. Our results also show that the activities of glycogen and enzyme histochemistry of liver graft and its recovery potency after reperfusion can serve as the important criteria to evaluate the quality of liver donors.
| 1. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 502] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Hines IN, Harada H, Wolf R, Grisham MB. Superoxide and post-ischemic liver injury: potential therapeutic target for liver transplantation. Curr Med Chem. 2003;10:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 414] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | He XS, Ma Y, Chen GH, Zhang JX, Wu JL, Liang YJ, Lin GY, Zhu ZY, Hu RD, Huang JF. The influence of warm ischemia injury on viability and posttransplantative outcome of liver graft from non-heart-beating donor in rats. Zhonghua YiXue ZaZhi. 2003;83:1236-1240. [PubMed] |
| 6. | Ma Y, He XS, Chen GH. Surgical technique of the model of orthotopic liver transplantation and prevention of operational complication in rats. Zhonghua Xianwei Waike Zazhi. 2003;26:45-47. |
| 7. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1993;93:64-68. |
| 8. | Sun JH, Zeng QH, Wu MC. Experience with orthotopic rat liver transplantation. Chin Med J (Engl). 1990;103:142-145. [PubMed] |
| 9. | Moench C, Moench K, Lohse AW, Thies JC, Otto G. Arterial back table pressure perfusion prevents ischemic biliary lesions after orthotopic liver transplantation. Chirurg. 2003;74:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Nowak G, Ungerstedt J, Wernerson A, Ungerstedt U, Ericzon BG. Hepatic cell membrane damage during cold preservation sensitizes liver grafts to rewarming injury. J Hepatobiliary Pancreat Surg. 2003;10:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Regueira FM, Espí A, Nwose P, Díez-Caballero A, Baixaulí J, Rotellar F, Olea J, Pardo F, Hernández-Lizoain JL, Cienfuegos JA. Comparison between two warm ischemic models in experimental liver transplantation in pigs. Transplant Proc. 2003;35:1591-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Belous A, Knox C, Nicoud IB, Pierce J, Anderson C, Pinson CW, Chari RS. Reversed activity of mitochondrial adenine nucleotide translocator in ischemia-reperfusion. Transplantation. 2003;75:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 15. | Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | He X, Ma Y, Chen G, Lin G, Wu J, Zhu Z, Huang J. Influence of warm ischemia injury on energy metabolism and survival of liver graft in rats. Zhonghua WaiKe ZaZhi. 2002;40:936-939. [PubMed] |
| 17. | Sato M, Ohkohchi N, Tsukamoto S, Koyamada N, Asakura T, Enomoto Y, Usuda M, Miyagi S, Okada A, Satomi S. Successful liver transplantation from agonal non-heart-beating donors in pigs. Transpl Int. 2003;16:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | He XS, Ma Y, Wu LW, Ju WQ, Chen GH, Hu RD, Huang JF. Influence of warm ischemia injury on hepatic functional status and survival of liver graft in rats. Hepatobiliary Pancreat Dis Int. 2003;2:504-508. [PubMed] |