Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2408
Revised: September 1, 2004
Accepted: December 16, 2004
Published online: April 28, 2005
AIM: To study the distribution and stability of antisense oligodeoxynucleotide (ASODN) in Walker-256 cells and their distribution in liver, lung and kidney tissues after being infused alone or mixed with lipiodol via hepatic artery in a rat liver tumor model.
METHODS: 5’-Isothiocyananate (FITC)-labeled vascular endothelial growth factor (VEGF) ASODN was added into Walker-256 cell culture media. Its distribution in cells was observed by fluorescence microscope at different time points. Walker-256 carcinosarcoma was transplanted into Wistar rat liver to establish a liver cancer model. 5’-FITC-labeled VEGF ASODN mixed with (mixed group, n = 6) or without (TAI group, n = 6) ultra-fluid lipiodol was administrated via hepatic artery. Frozen samples of liver, lung and kidney tissue were taken from rats after 1, 3 and 6 d, respectively. The distribution of ASODN was observed under fluorescent microscope.
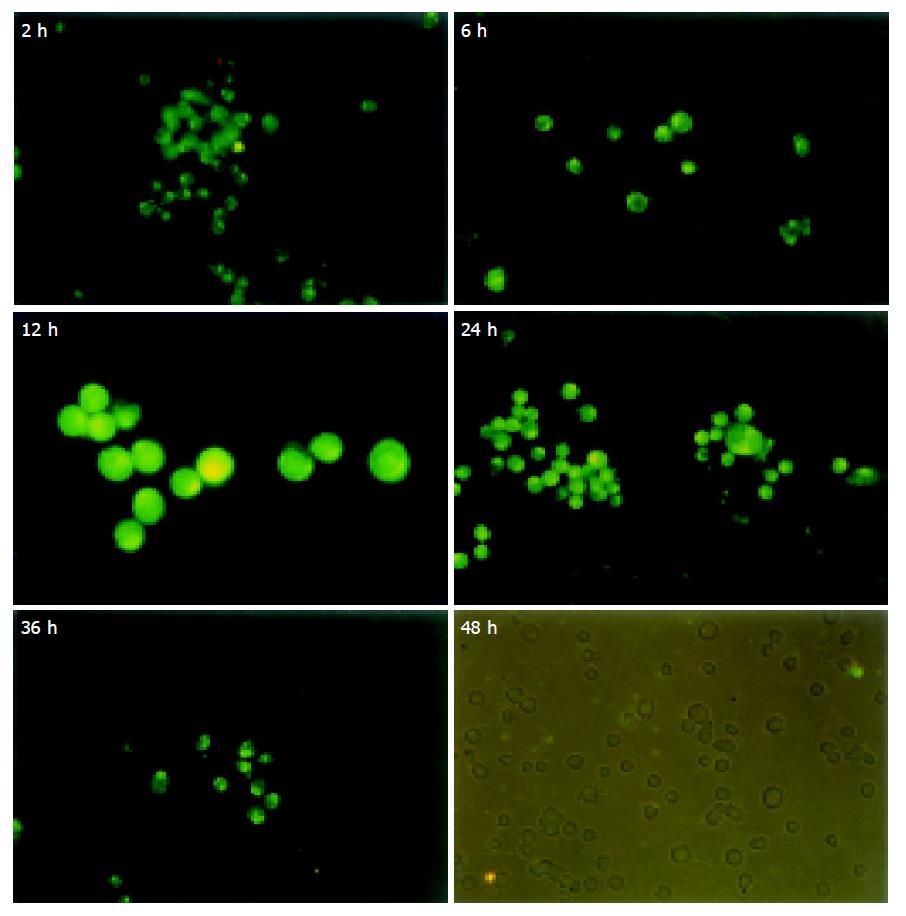
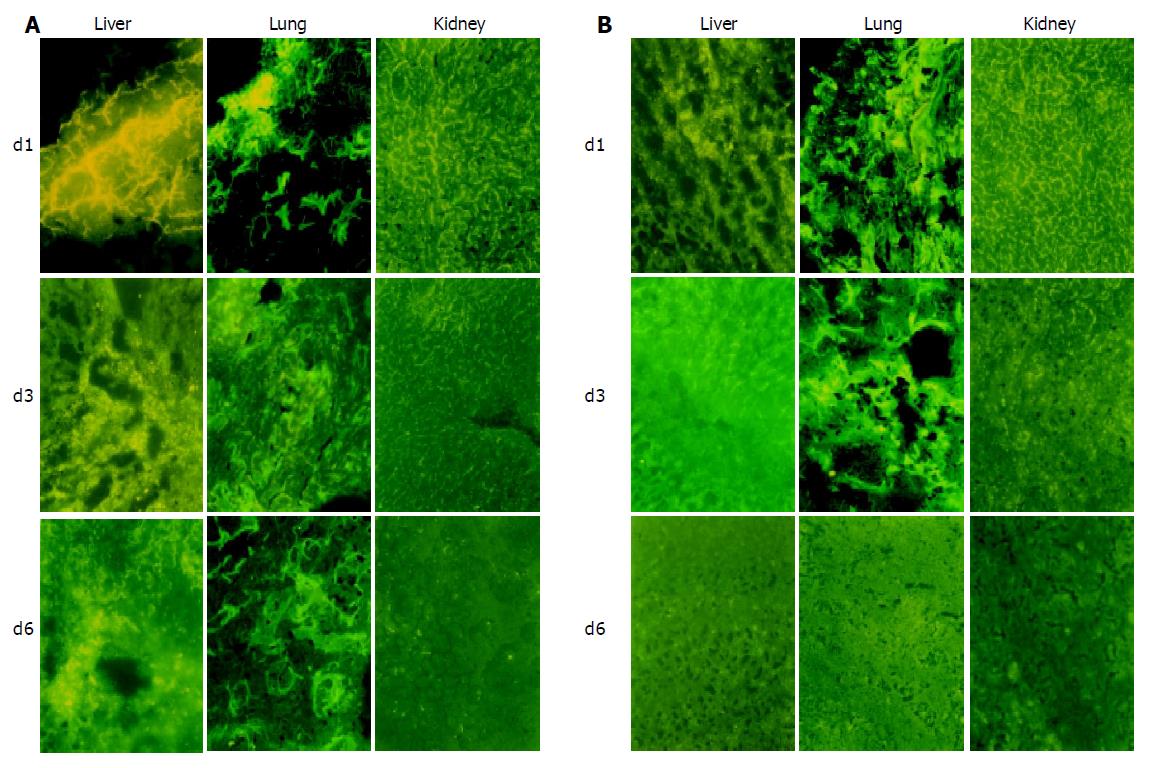
RESULTS: ASODN could enter cytoplasm within 2 h and nuclei within 6 h. Accumulation of ASODN reached the peak point in nuclei at 12 h, and then disappeared gradually. No fluorescence could be seen in cells at 48 h. In vivo experiment, on d 1 and 3 the fluorescence staining in liver was stronger in mixed group than in TAI group and more fluorescence could be detected in lung and kidney in TAI group than in mixed group. On d 6, no fluorescence could be detected in TAI group, but faint fluorescence could be seen in mixed group. ASODN could be seen in cancer cells and normal hepatic cells. In mixed group, ASODN was mainly distributed in liver tumor tissues.
CONCLUSION: ASODN can transfect Walker-256 cells. ASODN mixed with lipiodol infusion via hepatic artery can be used in the treatment of HCC.
- Citation: Wu HP, Feng GS, Tian Y. Hepatic artery infusion of antisense oligodeoxynucleotide and lipiodol mixture transfect liver cancer in rats. World J Gastroenterol 2005; 11(16): 2408-2412
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2408
Angiogenesis plays an important role in tumor progression and metastasis. One of the key mediators of angiogenesis is vascular endothelial growth factor (VEGF)[1-4]. It is overexpressed and secreted by the majority of human and animal tumors. Hepatocellular carcinoma (HCC) is one of the most common cancers in China. It is a hypervascular cancer and easy to metastasize. Recent studies indicate that VEGF is overexpressed in HCC tissues and positively related with progression and metastasis[5-7].
Antisense oligodeoxynucleotides (ASODNs) are short synthetic DNA molecules, their sequences are complementary to those present in specific target mRNA within cells. They can bind to target mRNA and block gene expression. They are becoming the focus of attention as a tool for down-regulation of gene expression that contributes to disease states[8]. It was reported that VEGF ASODN inhibits VEGF expression of cultured tumor cells and growth of implanted tumors in nude mice and is a potential drug to inhibit angiogenesis, prevent recurrence and metastasis of HCC[9-11]. However, there is no satisfactory method to deliver ASODN into the target tumor tissue and cells. Systemic administration of ASODN needs a high dosage, causes many side effects, and has no organ specificity for gene transfection[12-15]. Hepatic artery infusion (HAI) of ASODN can result in transfection organ or tumor tissue specificity; reduce the dosage and side effects. However, the time of ASODN action with tumor cells is transient and the transfection efficiency is still low.
Lipiodol is a common liquid embolic agent for transcatheter hepatic artery embolization for HCC. It stays in tumor tissue for a long period of time (several months), is absorbed by tumor cells[16,17], and acts as a carrier of chemotherapeutic agents to maximize the concentration and exposure time in the tumor. We assumed that ASODN and lipiodol mixture infused via hepatic artery could enhance its transfection efficiency. In this study, we compared the different distributions of ASODN in Walker-256 carcinosarcoma liver transplantation model after infusion of ASODN alone or mixed with lipiodol via hepatic artery.
Phosphorothioate ASODN with 5’ end labeled with fluorescein isothiocyanate (FITC) was synthesized by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd. Its sequence is 5’-GCAGTAGCTGCGCTGATAGCGC-3’, complementary to the exon 3 area of VEGF mRNA.
Walker-256 carcinosarcoma cells were purchased from China Center for Type Culture Collection. After recovery, the cells were inoculated into the abdominal cavity of male pathogen-free Wistar rats, weighing 100-120 g (Department of Experimental Animals, Tongji Medical College). Three days later, 0.2 mL of cancerous ascites was aspirated and cultured in RPMI-1640 containing 5 mL/L fetal calf serum (FCS) (Gibco, Grand Island, NY) and equilibrated with 950 mL/L air and 50 mL/L CO2. Cells were passaged every 2 d. After the 3rd passage, cells were seeded into a 24-well plate at 1×103/well (100 μL/well), cultured with 33 μg (20 μL) FITC-ASODN at 37 °C in a humidified atmosphere containing 950 mL/L air and 50 mL/L CO2. At 2, 6, 12, 24, 36 and 48 h, the cells were harvested, washed with non-serum medium thrice, dropped onto a slide, and then the FITC-ASODN cellular distribution was observed under a fluorescence microscope (Olympus, Japan).
Tumor implantation was performed using a technique described by Li et al. Briefly, Walker-256 carcinosarcoma cells were inoculated subcutaneously into the right flank of Wistar rats with 1×107 tumor cells in approximately 0.1 mL of cell suspension. The tumor was palpable 7 d after inoculation. Fresh tumor tissue was isolated and cut into 1-mm3 sections. The recipient animal was laparotomized through a midline abdominal incision under intraperitoneal anesthesia with 1% pentobarbital sodium (30 mg/kg body weight). The left lateral lobe of the liver was protruded out of the abdominal cavity and a subcapsular tunnel about 3-5 mm in depth was made by a fine-point tweezers. Then a solid tumor fragment was inserted into the subcapsular tunnel and fixed with a small piece of gelfoam on the liver surface. The abdominal cavity was closed, and the rat was bred. One week later, a transplanted tumor 0.5-1 cm in diameter grew in the left lobe of liver.
Ten days after the implantation, another midline abdominal incision was made under intraperitoneal anesthesia. The common hepatic artery, gastroduodenal artery and right hepatic artery were isolated under a binocular operative microscope (Suzhou Medical Instruments Factory, Jiangsu, China). Through an arteriotomy of the gastroduodenal artery, the gastroduodenal artery was catheterized retrograde for drug infusion with a Portex PE10 tube (inner diameter 0.28 mm, external diameter 0.61 mm, Neolab, Germany) and temporarily fixed by a suture.
Twelve tumor-bearing rats were divided into TAI group and mixed group, six rats each. TAI group received 165 μg FITC-ASODN diluted with 0.2 mL PBS and mixed group received 165 μg FITC-ASODN mixed with 0.2 mL lipiodol (Lipiodol UltraFluid, Andre Guerbet, Aulnay-sous-Bois, France). The drugs were infused retrograde into the gastroduodenal artery through the PE10 tube, while the common hepatic artery and right hepatic artery were temporarily ligated. Ten minutes after drug infusion, the tube was pulled out, the gastroduodenal artery was ligated, the common hepatic artery and right hepatic artery were untied and the abdominal wall was closed.
On d 1, 3 and 6 after drug administration, two animals in each group were killed at each time point. The liver tumor tissue, adjacent normal liver tissue, lung and kidney tissue samples were excised and frozen in liquid nitrogen and stored at -70 °C. Frozen sections (5 μm in thickness) were cut and observed under a fluorescence microscope.
At 2 h after Walker-256 cells were cultured with FITC-ASODN, granular fluorescence was detected in cytoplasm, and no fluorescences were detected in nuclei. At 6 h fluorescences in cytoplasm became denser and granular fluorescences were seen in nuclei. At 12 h fluorescences in cytoplasm and nuclei reached the peak. Then fluorescences disappeared gradually. No fluorescences could be seen in cells at 48 h (Figure 1).
In mixed group, on d 1, fluorescences were detected mainly in liver tumor tissues and heavily stained with deep-yellow color. The delimitation of cells was hard to identify. Some peri-tumor zone also contained fluorescences. In lung and kidney tissues, the fluorescence was stained lightly. Piece-like fluorescence stain could be detected in some small areas of the lung tissues. On d 3, fluorescences in tumor and peri-tumor tissues became less, were mainly distributed in tumor tissue and could be found in most tumor cells. On d 6, fluorescences in tumor and peri-tumor tissues became further less, but fluorescences were still seen. Faint fluorescences were seen in lung and kidney on d 3 and 6 (Figure 2A).
In TAI group, fluorescences were dense in liver tumor and peri-tumor tissue, lung and kidney tissues on d 1, and then decreased gradually. No fluorescence was seen on d 6. The fluorescences in liver tumor and peri-tumor tissue in TAI group were much less than those in mixed group at each time point (Figure 2B).
ASODN is a new therapeutic agent for HCC treatment. It binds specifically to mRNA via Watson-Crick base pair interaction and blocks the translation of corresponding proteins[9]. It can penetrate cell membrane and achieve appropriate concentration in the correct intracellular compartment (cytoplasm or nucleus)[18]. Tumor cell is the target cell of most designed ASODNs, such as VEGF ASODN. How to efficiently deliver ASODN to liver tumor cells remains to be solved[19]. Systemic intravenous injection is the routine route for ASODN administration. Studies of the distribution and metabolism of ASODN revealed that ASODN delivery has no organ specificity, ASODN stays in liver transiently, and high dose is required for transfection of hepatic cells[20-23]. Graham et al[24,25] reported that after a 10 mg/kg ASODN bolus, intracellular drug levels in liver reached the peak at 8 h and diminished significantly thereafter. Nonparenchymal (Kupffer and endothelial) cells contain approximately 80% of the total organ cellular dose, while the remaining 20% is associated with hepatocytes. At doses lower than 25 mg/kg, hepatocytes contain significantly less drug with no detectable ASODN in nuclei. Doses at or above 25 mg/kg appear to saturate nonparenchymal cell types, whereas hepatocytes continue to accumulate drugs in all cellular compartments, including nuclei. A higher dose of ODN may result in more side effects such as dose-dependent hypotension, complement activation, and transient prolongation of thromboplastin time[12-15]. Transcatheter artery infusion (TAI) can enhance the concentration of therapeutic gene, realize gene transfection in target organ selectively, and reduce dose and side effects. This administration route has been widely used in experimental and clinical gene therapies for brain tumor[26], coronary artery occlusive diseases[27], etc. However, as for the liver tumor treatment, the action ASODN on HCC cells is still transient, the transfection efficiency is still low, and the in vivo distribution of ASODN in liver and other organs after HAI has not been reported.
Most HCCs are hypervascular tumors. Their blood supply mainly comes from hepatic artery. Lipiodol can selectively accumulate in HCC when it is injected into the hepatic artery. The mechanism of selective retention of lipiodol in HCC includes the “siphoning effect” of tumor vessels resulting in lipiodol flowing into tumor vessels, the electrostatic difference between lipiodol and cancerous endothelia, transcapillary leak coupled with lack of lymphatic and Kupffer cell’s clearance of lipiodol in HCC, membranous attachment of lipiodol to tumor cells, pinocytosis of lipiodol by tumor cells[18]. Lipiodol is a common liquid embolic agent and used as a carrier of chemotherapeutic agents for transcatheter artery chemoembolization of HCC.
In this study, ex vivo cell culture experiment indicated that ASODN could enter the cytoplasm and nuclei of Walker-256 cell and stay in a short period of time (about 36 h). In in vivo animal experiment, we found that ASODN mixed with lipiodol could transfect and enter into tumor cells and adjacent normal hepatic cells, mainly in tumor cells, and stay for more than 5 d. However, in TAI group, the distribution of ASODN in tumor and adjacent tissues showed no difference, and the retention of ASODN in liver tissue was less and shorter (about 3 d) than that in mixed group. The concentration of ASODN in lung and kidney tissue in mixed group was lower than that in TAI group on d 1. These findings indicate that ASODN can transfect normal hepatic cells and tumor cells without specificity, lipiodol can act as a carrier of ASODN to make it mainly distribute in tumor tissue, ASODN mixed with lipiodol can slowly diffuse into tumor tissue, act a longer period of time with tumor cells, thus enhancing the transfection rate and minimizing the distribution of ASODN in organs outside the liver.
In mixed group, piece-like fluorescence stained areas were seen in liver tissue on d 1. This is because FITC-ASODN does not release from lipiodol at that time. We also found piece-like fluorescence stain in some area of the lung on d 1. This is because the mixed ASODN and lipiodol enter the pulmonary artery and stay there.
ASODN mixed with lipiodol can enter tumor cells, but its mechanism is not clear. It may be due to the following points: (1) ASODN can release from lipiodol; (2) tumor vessels are immature, lack tunica media, and the gaps between endothelial cells are wide, leading to leak of ASODN and lipiodol into extracellular space easily; (3) lipiodol and ASODN can be absorbed by tumor cells.
In conclusion, ASODN mixed with lipiodol infusion via hepatic artery can be used in the treatment of HCC.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ziemer LS, Koch CJ, Maity A, Magarelli DP, Horan AM, Evans SM. Hypoxia and VEGF mRNA expression in human tumors. Neoplasia. 2001;3:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, Van Marck E. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis. 2001;4:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Cascinu S, Graziano F, Catalano V, Staccioli MP, Barni S, Giordani P, Rossi MC, Baldelli AM, Muretto P, Valenti A. Differences of vascular endothelial growth factor (VEGF) expression between liver and abdominal metastases from colon cancer. Implications for the treatment with VEGF inhibitors. Clin Exp Metastasis. 2000;18:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Hirohashi K, Yamamoto T, Uenishi T, Ogawa M, Sakabe K, Takemura S, Shuto T, Tanaka H, Kubo S, Kinoshita H. CD44 and VEGF expression in extrahepatic metastasis of human hepatocellular carcinoma. Hepatogastroenterology. 2004;51:1121-1123. [PubMed] |
| 7. | Zhao J, Hu J, Cai J, Yang X, Yang Z. Vascular endothelial growth factor expression in serum of patients with hepatocellular carcinoma. Chin Med J (Engl). 2003;116:772-776. [PubMed] |
| 8. | Narayanan R, Akhtar S. Antisense therapy. Curr Opin Oncol. 1996;8:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Yang PY, Rui YC, Jin YX, Li TJ, Qiu Y, Zhang L, Wang JS. Antisense oligodeoxynucleotide inhibits vascular endothelial growth factor expression in U937 foam cells. Acta Pharmacol Sin. 2003;24:610-614. [PubMed] |
| 10. | Gong BD, Luo W, Du FT, Ye RM, Liu JM, Yu CG, Zou YQ, Zhang JX. Inhibitory effects of antisense oligonucleotides on VEGF gene expression by human hepatocellular carcinoma cells. Zhonghua GanZangBing ZaZhi. 2004;12:35-37. [PubMed] |
| 11. | Zhu J, Huang J, Chen Y. Effect of PCNA antisense oligooxynucleotides and VEGF antisense oligoxynucleotides on growth of hepatocellular carcinoma transplanted in nude mice. Zhonghua WaiKe ZaZhi. 2001;39:875-877. [PubMed] |
| 12. | Iversen PL, Copple BL, Tewary HK. Pharmacology and toxicology of phosphorothioate oligonucleotides in the mouse, rat, monkey and man. Toxicol Lett. 1995;82-83:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Leeds JM, Henry SP, Bistner S, Scherrill S, Williams K, Levin AA. Pharmacokinetics of an antisense oligonucleotide injected intravitreally in monkeys. Drug Metab Dispos. 1998;26:670-675. [PubMed] |
| 14. | Tolcher AW, Reyno L, Venner PM, Ernst SD, Moore M, Geary RS, Chi K, Hall S, Walsh W, Dorr A. A randomized phase II and pharmacokinetic study of the antisense oligonucleotides ISIS 3521 and ISIS 5132 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2002;8:2530-2535. [PubMed] |
| 15. | Webb MS, Tortora N, Cremese M, Kozlowska H, Blaquiere M, Devine DV, Kornbrust DJ. Toxicity and toxicokinetics of a phosphorothioate oligonucleotide against the c-myc oncogene in cynomolgus monkeys. Antisense Nucleic Acid Drug Dev. 2001;11:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Han GH, Guo QL, Huang GS, Guo YL. Distribution of lipiodol in hepatocellular carcinoma after hepatic arterial injection and its significance. Zhonghua Fangshexue Zazhi. 1993;27:828-831. |
| 17. | Kan Z, Sato M, Ivancev K, Uchida B, Hedgpeth P, Lunderquist A, Rosch J, Yamada R. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Butler M, Stecker K, Bennett CF. Cellular distribution of phosphorothioate oligodeoxynucleotides in normal rodent tissues. Lab Invest. 1997;77:379-388. [PubMed] |
| 19. | Lappalainen K, Miettinen R, Kellokoski J, Jääskeläinen I, Syrjänen S. Intracellular distribution of oligonucleotides delivered by cationic liposomes: light and electron microscopic study. J Histochem Cytochem. 1997;45:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Geary RS, Yu RZ, Watanabe T, Henry SP, Hardee GE, Chappell A, Matson J, Sasmor H, Cummins L, Levin AA. Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2'-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos. 2003;31:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Bijsterbosch MK, Manoharan M, Dorland R, Van Veghel R, Biessen EA, Van Berkel TJ. bis-Cholesteryl-conjugated phosphorothioate oligodeoxynucleotides are highly selectively taken up by the liver. J Pharmacol Exp Ther. 2002;302:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ponnappa BC, Dey I, Tu GC, Zhou F, Aini M, Cao QN, Israel Y. In vivo delivery of antisense oligonucleotides in pH-sensitive liposomes inhibits lipopolysaccharide-induced production of tumor necrosis factor-alpha in rats. J Pharmacol Exp Ther. 2001;297:1129-1136. [PubMed] |
| 23. | Zhang R, Iyer RP, Yu D, Tan W, Zhang X, Lu Z, Zhao H, Agrawal S. Pharmacokinetics and tissue disposition of a chimeric oligodeoxynucleoside phosphorothioate in rats after intravenous administration. J Pharmacol Exp Ther. 1996;278:971-979. [PubMed] |
| 24. | Graham MJ, Crooke ST, Monteith DK, Cooper SR, Lemonidis KM, Stecker KK, Martin MJ, Crooke RM. In vivo distribution and metabolism of a phosphorothioate oligonucleotide within rat liver after intravenous administration. J Pharmacol Exp Ther. 1998;286:447-458. [PubMed] |
| 25. | Graham MJ, Crooke ST, Lemonidis KM, Gaus HJ, Templin MV, Crooke RM. Hepatic distribution of a phosphorothioate oligodeoxynucleotide within rodents following intravenous administration. Biochem Pharmacol. 2001;62:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Koga H, Inamura T, Ikezaki K, Samoto K, Matsukado K, Fukui M. Selective transvascular delivery of oligodeoxynucleotides to experimental brain tumors. J Neurooncol. 1999;43:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Matsushita H, Morishita R, Aoki M, Tomita N, Taniyama Y, Nakagami H, Shimozato T, Higaki J, Kaneda Y, Ogihara T. Transfection of antisense p53 tumor suppressor gene oligodeoxynucleotides into rat carotid artery results in abnormal growth of vascular smooth muscle cells. Circulation. 2000;101:1447-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |