Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2402
Revised: July 10, 2004
Accepted: September 19, 2004
Published online: April 28, 2005
AIM: To investigate the value of contrast-enhanced C3-MODE technology in differentiating malignant nodules of liver from the benign ones.
METHODS: Forty-six nodules in 36 patients (29 men and 7 women) were studied by contrast-enhanced C3-MODE technology and contrast-enhanced CT in 1 wk before the biopsy or operation. A low MI monitor and a high MI flash imaging were intermittently performed. After the injection of contrast agent, the period from 10 to 30 s and the time later than 100 s were respectively defined as early arterial phase and the late phase. The vascularities of the liver nodules in the two phases were combined for differential diagnosis. Corresponding to the pathological diagnosis, the accuracy, sensitivity and specificity of contrast-enhanced C3-MODE technology were compared to those of contrast-enhanced CT.
RESULTS: By C3-MODE technology, 33 of the 46 liver nodules were demonstrated as defected area in the late phase and were diagnosed as malignant tumors. Of them, 28 with hypervascularity in the early arterial phase were assessed as hepatocellular carcinoma, the other five nodules with rim-like enhancement in the early arterial phase were diagnosed as metastatic tumors. Thirteen nodules were shown as iso or hypervascularity in the late phase as well as centripetal filling in the early arterial phase and we made a diagnosis of hemangioma. Corresponding to the pathological results, the sensitivity, specificity and accuracy of contrast-enhanced C3-MODE technology in differentiating malignant and benign nodules in the liver were 97.0%, 92.3% and 95.7%, respectively. With comparison to those of contrast CT (sensitivity, 94.1%; specificity, 91.7%; accuracy, 93.5%), the difference was not significant.
CONCLUSION: Contrast-enhanced C3-MODE technology can effectively differentiate malignant liver tumors from the benign nodules. It highly agrees diagnostically with the pathology. We suggest that it provides a new approach for differential diagnosis of liver nodules in addition to contrast-enhanced CT.
- Citation: Luo BM, Wen YL, Yang HY, Zhi H, Ou B, Ma JH, Pan JS, Dai XN. Differentiation between malignant and benign nodules in the liver: Use of contrast C3-MODE technology. World J Gastroenterol 2005; 11(16): 2402-2407
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2402
With the development of ultrasonography, small lesions in liver can be sensitively detected[1]. However, because of the similar appearance in B-mode image, it is difficult for B-mode ultrasonography to differentiate the malignant from the benign ones.
The intranodular blood supply is the base of differential diagnosis of hepatic nodules. Color Doppler imaging (CDI) and power Doppler imaging (PDI) are quite useful in some settings for detecting the intranodular blood signals[2,3]. Unfortunately, the ability is not satisfying due to the low sensitivity of CDI and PDI to weak blood signals, and the differential diagnosis of nodular hepatic lesions should be on the basis of other imaging approaches, such as three-phase dynamic CT, MRI or digital subtraction angiography[4,5]. As ultrasonography is often the first choice of scanning in assessment of nodular hepatic lesions because of its noninvasiveness and cheapness, new technologies of ultrasound do great effort for revealing the difference between malignant and benign lesions. Contrast-enhanced ultrasound is one of those newly developed technologies. With use of contrast agent and some special contrast-enhanced technique, the ability of differentiation on ultrasound has been greatly developed[6-14].
Combined Contrast Chain procession (C3-MODE technology) has been developed in recent years. Based on the delayed transmission and the technology that modify the signals of different phase and frequency in different ways, C3-MODE technology can use not only the sub-harmonic, second harmonic and ultra-harmonic signals, but also the fundamental signals which come from the microbubbles. As a result, C3-MODE technology theoretically has high sensitivity and specificity to the signals produced by microbubbles. This study was planned to investigate the detectability of contrast-enhanced C3-MODE technology on intranodular blood signals of nodular hepatic lesions, and its ability in differentiating malignant from benign nodular lesions of liver.
From March to October 2002, 36 patients with 46 nodular lesions of liver were enrolled into this study. Oral informed consent from each patient was obtained by explaining the purpose of the study before contrast-enhanced ultrasonography was performed. Of the patients, 29 were men and 7 were women. The ages ranged from 29 to 76 years (mean±SD, 52±12 years). Fifteen of them had B type hepatitis and the others did not have any type of hepatitis. Twenty of the patients had single lesion and the other ten had double lesions in the liver. The maximal diameter of the lesion was recorded according to ultrasonography and ranged from 0.49 cm to 5.72 cm (mean±SD, 2.67±1.26 cm). Thirty-three of the lesions were less than 3.0 cm in maximal diameter (mean±SD, 1.96±0.74 cm). The depths of the lesions (the distance from the surface of the skin to the far edge of the lesions) were from 3.6 cm to 12 cm (mean±SD, 7.6±2.37 cm). All patients were diagnosed with C3-MODE technology, contrast-enhanced CT, MRI and/or DSA. The final diagnosis for each nodule was confirmed by ultrasound-guided biopsy or pathological examination after operation.
Ultrasonography was performed a week before biopsy or operation. The used machine was Esaote Technos DU6 (Esaote Co., Italy). A convex-arrayed transducer was used with the frequency of 2.5 MHz. Before contrast-enhanced C3-MODE technology was performed, the lesions were scanned with fundamental B-mode and CDI to choose a good plane and posture for researching. During this time, the size, echo type and the depth of the lesion were recorded. C3-MODE technology was started with a low MI of 0.2-0.3. After administration of contrast agent, flash imaging was emitted intermittently with a high MI of 1.0-1.2. Focus was located in the deep level of the lesion. From the time about 10 s after the bolus injection of Levovist (early arterial phase), the flash imaging was performed 3-5 times with an interval of about 5 s. Then low MI C3-MODE technology was scanned to monitor the lesion. In the late phase (after 100 s from the administration of the contrast agent), flash imaging was emitted twice with an interval of 10 s. All ultrasonography were performed by the same sonographer to minimize the bias. The information of ultrasound examination was stored on magnetic optical disks and digital videotapes.
Levovist was used with a concentration of 300 mg/mL, which was obtained by adding 7 mL sterile water into 2.5 g Levovist microparticles. Eight milliliters of the contrast agent was injected as a bolus via an antecubital venous with the speed of 1 mL/s, then, flash with 5 mL of normal saline. If there were two nodules in the liver, two vials of Levovist were used. Each of the contrast agents was injected separated by an interval of more than 10 min.
All the contrast-enhanced C3-MODE technology studies were performed by the same operators for decreasing the variation. The contrast-enhanced patterns were assessed by three experienced sonographers who did not know the results from contrast-enhanced CT and pathological diagnosis. The contrast-enhanced pattern of the lesions in early arterial phase and late phase were assessed with comparison to the liver parenchyma around the lesions. After administration of Levovist, compared with peripheral liver tissue, hyperechoic or iso-echoic pattern was considered to be positive enhancement. In contrast, hypoechoic pattern was defined as negative enhancement.
Contrast-enhanced CT was performed a week before biopsy or operation. Siemens Somatom AR. T (Siemens, Berlin, German) was used. A plain scanning was obtained before contrast agent was used. The used contrast agent was 100 mL of Iopromide 300 (Schering, Guangzhou, China). About 25 s after the administration of the contrast agent, contrast-enhanced CT scanning was obtained. On contrast-enhanced CT scanning, a high attenuation or iso-attenuation of the hepatic nodule was assessed as positive intranodular vascularity. A low attenuation of the nodule was considered to be negative intranodular vascularity. All CT scanning were reviewed by two experienced radiologists who did not know the results on contrast-enhanced C3-MODE technology.
The sensitivity, specificity and accuracy of each approach for differentiating malignant and benign lesions were assessed corresponding to pathological diagnosis. When the ability of contrast-enhanced C3-MODE technology in differentiating malignant and benign lesions was compared to that of contrast-enhanced CT, the χ2-square test was used. When P value was less than 0.05, the difference was considered to be significant.
The diagnosis made by contrast-enhanced C3-MODE technology and pathological diagnoses are shown in Table 1.
| C3-MODE | Pathological diagnosis | |||
| HCC | Metastatic tumor | Hemangioma | Lipid degeneration | |
| HCC | 27 | 0 | 1 | 0 |
| Metastatic tumor | 0 | 5 | 0 | 0 |
| Hemangioma | 0 | 1 | 9 | 3 |
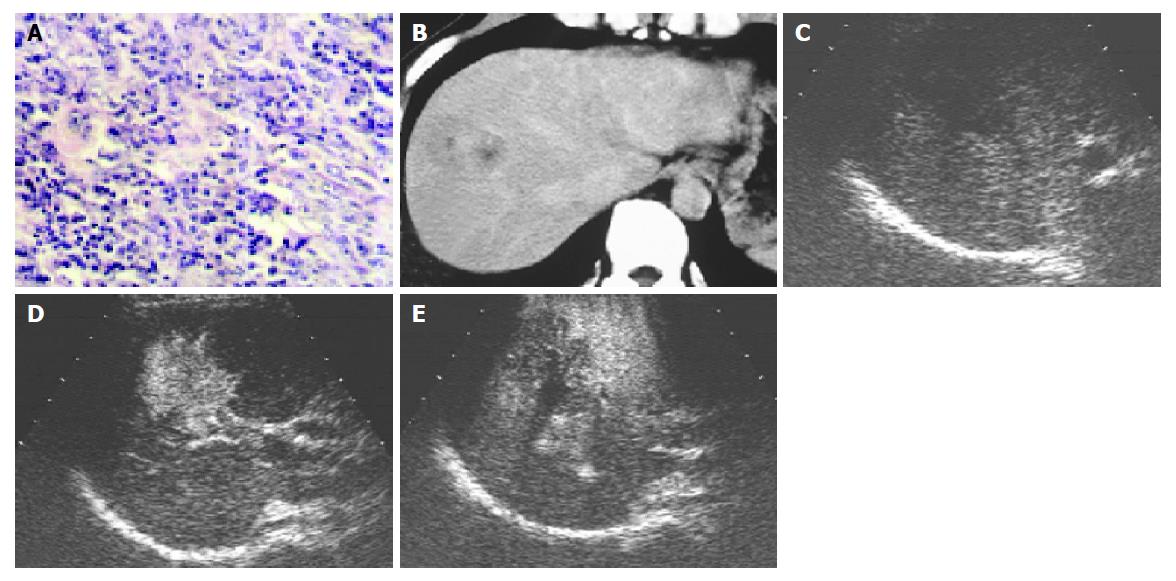
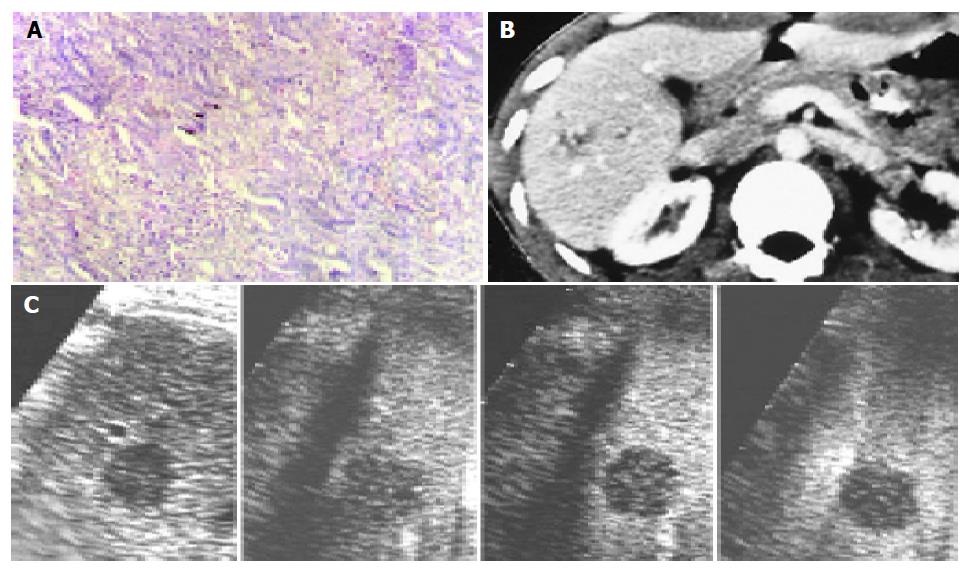
On contrast-enhanced C3-MODE technology, 33 of the 46 nodules were demonstrated as defected area in the late phase and were assessed to be malignant tumors. Of them, 28 nodules showed intranodular vascularity, which appeared from the edge to the center of the nodules, and assessed to be hypervascular tumors in the early arterial phase and HCC was diagnosed (Figure 1). The other five nodules showed rim-like enhancement in the early arterial phase and the diagnosis of metastasis was made (Figure 2). Corresponding to the pathological diagnosis, the positive and negative predictive value to hepatic malignant tumor of contrast-enhanced C3-MODE technology was 97.0% (32/33) and 92.3% (12/13), respectively.
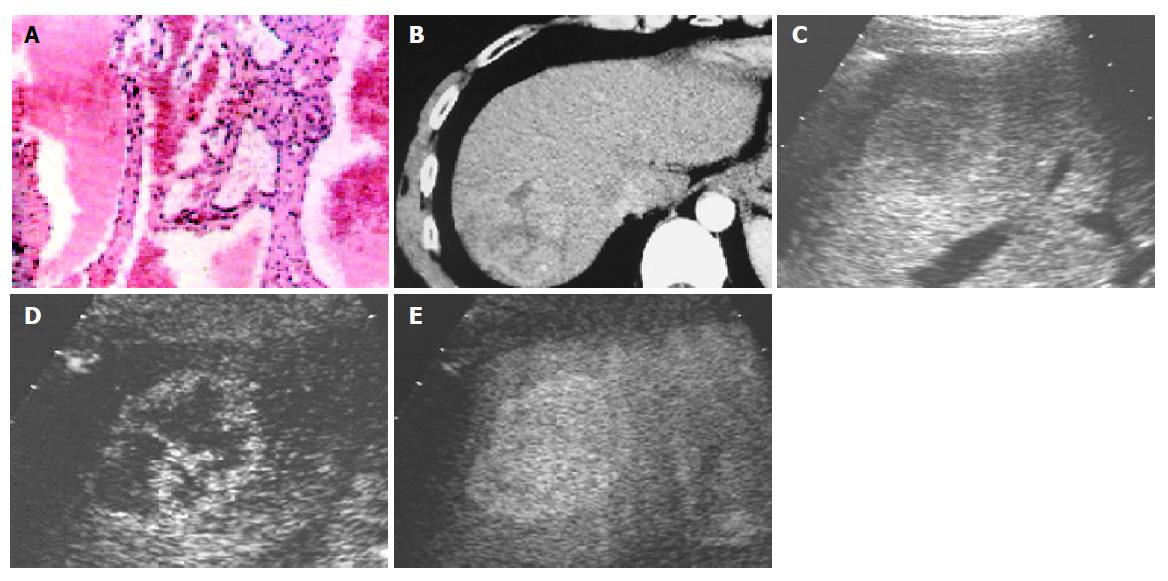
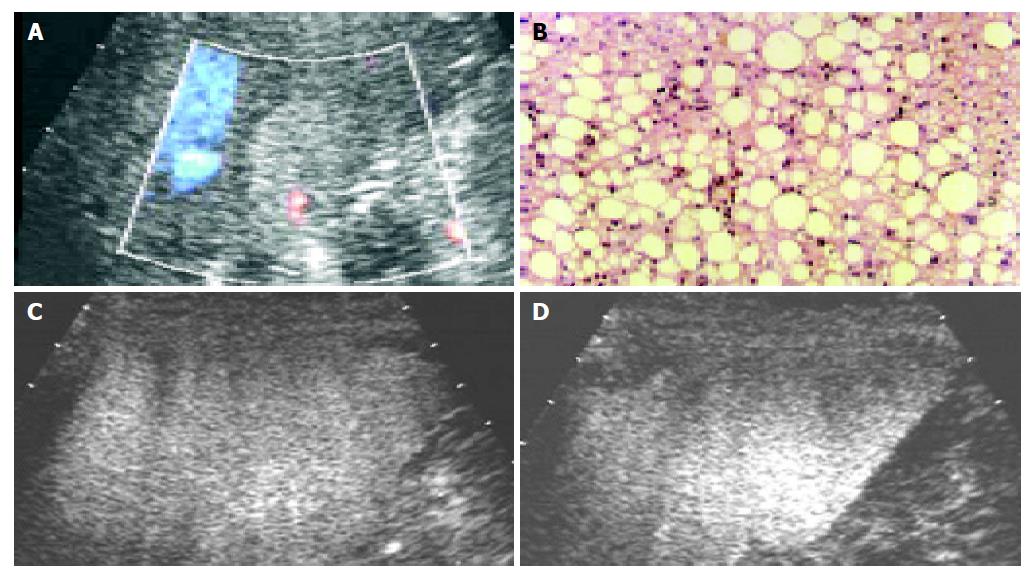
Using contrast-enhanced C3-MODE technology, 13 nodules were found with spot-like or wool-cotton enhancement and centripetal filling in the early arterial phase followed by long-time enhancement which lasted till the late phase. All of them were assessed to be hemangioma (Figure 3). According to the pathological diagnosis, the three fatty degenerative nodules (Figure 4) and one metastatic tumor were falsely diagnosed.
Corresponding to the results of pathological diagnosis, the sensitivity, specificity and accuracy of contrast-enhanced C3-MODE technology on malignant hepatic tumors were 97.0%, 92.3% and 95.7%, respectively (Table 2).
| Pathological diagnosis | Sensitivity | Specificity | Accuracy | |||
| Malignant | Benign | |||||
| C3-MODE | Malignant | 32 | 1 | 97.0% | 92.3% | 95.7% |
| Benign | 1 | 12 | ||||
| CT | Malignant | 32 | 2 | 94.1% | 91.7% | 93.5% |
| Benign | 1 | 11 | ||||
| Total | 33 | 13 | ||||
The diagnosis made by adopting contrast-enhanced CT is shown in Table 2. One HCC was falsely diagnosed to be cystic change on the basis of its low attenuation on contrast-enhanced CT. In addition, two of the fatty degenerative nodules were assessed to be HCC by contrast-enhanced CT. Hence, the diagnostic sensitivity, specificity and accuracy of contrast-enhanced CT to malignant hepatic tumors was 94.1%, 91.7% and 93.5%, respectively (Table 2). There was no significant difference between the ability of contrast-enhanced C3-MODE technology and contrast-enhanced CT on differential diagnosis of nodular hepatic nodules.
Contrast-enhanced ultrasonography has been developed rapidly in recent years. From fundamental CDI to second harmonic imaging, the detectability of contrast-enhanced ultrasound to the intratumoral vascularity of hepatic tumors increased significantly[6,7]. However, the relatively low sensitivity to contrast-enhanced signals of second harmonic imaging limited its use in clinical settings. Many kinds of new technologies were developed for higher detection of intratumoral vascularities of hepatic tumors for differential diagnosis[10,11]. Unfortunately, as the contrast agent was being injected via a peripheral venous, the concentration of the contrast agent decreased to 5% of the primary one when it reached the target organs because of the dilution of blood, attached by tissue or destroyed en route[15,16]. To increase the efficiency in utilization of the signals from microbubbles may be an effective way to increase the sensitivity of ultrasound. As reported, many new harmonic technologies greatly increased the detectability to harmonic signals by different mechanisms[17,18]. However, they ignore the fundamental signals scattered from microbubbles, which is the strongest part of the signals from the microbubbles. To effectively use this part of the signals may be a useful way to increase the sensitivity of ultrasound to the contrast-enhanced signals. On the basis of the techniques of delayed emission method, time and dimension double manager technique and contrast moving scan (low MI monitor and high MI flash), C3-MODE technology was recently developed. When it receives the signals from the contrast agent, C3-MODE technology catches not only the second harmonic signals, but also the sub-harmonic, ultra-harmonic and fundamental signals from the microbubbles. As a result, more signals scattered by microbubbles were used. Theoretically, a higher resolution and sensitivity could be obtained.
In this study, C3-MODE technology was performed with double mode, a low and a high MI mode. It is convenient to change the mode by pushing a small button. Low MI image can observe the lesion continuously without destroying the microbubbles of the contrast agent, and high MI image can demonstrate the flash image by destroying the microbubbles. So we can monitor the lesion on low MI image which helps us from not losing the target, and observe the contrast-enhanced effect on high MI image.
In this study, we differentiate malignant tumors from benign ones according to the perfusion defect in the late phase on contrast-enhanced C3-MODE technology. Thirty-two (69.6%) of the 46 nodules that showed perfusion defect in the late phase were proved to be malignant tumors, in this study. The specificity was as high as 97.0% and sensitivity was 92.3%. In contrast, those that showed high echo or iso-echo in the late phase were considered to be benign nodules. The diagnostic sensitivity and specificity were comparable to those of contrast-enhanced CT. With the help of the appearance in the early arterial phase, C3-MODE technology can effectively differentiate HCC from metastatic tumors. The diagnoses were highly agreeable with the pathological diagnosis.
On C3-MODE technology, one metastatic tumor was falsely diagnosed to be hemangioma and one hemangioma was falsely assessed to be HCC. This may be due to the high echo appearance of them on B-mode image, which disturbed the assessment of contrast-enhanced image. Other metastatic tumors were shown with rim-like enhancement in the early arterial phase and perfusion defect in the late phase. It may due to hypovascularity of these tumors which originated from the rectum.
In 13 benign nodules, three nodules of lipid degeneration were diagnosed to be hemangioma on C3-MODE technology. It may be due to the high echo of the nodules and the loss of experience of its hemodynamics. By reviewing the video, we found that homogenous enhancement of the nodules occurred in the early arterial phase but not in the cotton-wool pattern.
The relatively small group and fewer kinds of the nodules are limitations of this study. In addition, C3-MODE technology is still a high MI contrast-enhanced technology. It observes the contrast-enhanced effect mainly on the basis of high MI flash imaging. As the flash image is obtained, the microbubbles were destroyed rapidly. As a result, we cannot observe the contrast-enhancement continuously.
In conclusion, C3-MODE technology can effectively depict the blood supply of hepatic nodules in the early arterial phase and the late phase by using a double mode system. It gets a high diagnostic agreement with pathological diagnosis in differential diagnosis of nodular hepatic lesions. We suggest that it provides a new approach for differentiating the malignant tumors from the benign lesions in the liver.
| 1. | Menu Y. Hepatocellular carcinoma: radiological findings. Hepatogastroenterology. 1998;45 Suppl 3:1232-1235. [PubMed] |
| 2. | Tanaka S, Kitamura T, Fujita M, Nakanishi K, Okuda S. Color Doppler flow imaging of liver tumors. AJR Am J Roentgenol. 1990;154:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 155] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Kawasaki T, Itani T, Nakase H, Mimura J, Komori H, Sugimoto K. Power Doppler imaging of hepatic tumours: differential diagnosis between hepatocellular carcinoma and metastatic adenocarcinoma. J Gastroenterol Hepatol. 1998;13:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Kudo M. Imaging diagnosis of hepatocellular carcinoma and premalignant/borderline lesions. Semin Liver Dis. 1999;19:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Moriyama N, Takayama T, Yamasaki S, Sakamoto M, Hirohashi S. Early hepatocellular carcinoma: appearance at CT during arterial portography and CT arteriography with pathologic correlation. Radiology. 1995;194:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Fujimoto M, Moriyasu F, Nishikawa K, Nada T, Okuma M. Color Doppler sonography of hepatic tumors with a galactose-based contrast agent: correlation with angiographic findings. AJR Am J Roentgenol. 1994;163:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Wen YL, Kudo M, Minami Y, Chung H, Suetomi Y, Onda H, Kitano M, Kawasaki T, Maekawa K. Value of new contrast harmonic technique for detecting tumor vascularity in hepatocellular carcinoma: preliminary results. J Med Ultrasonics. 2003;30:85-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Wen YL, Kudo M, Minami Y, Chung H, Suetomi Y, Onda H, Kitano M, Kawasaki T, Maekawa K. Contrast-enhanced agent detection imaging: early experience in hepatocellular carcinoma. J Med Ultrasonics. 2003;30:77-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Wen YL, Kudo M, Maekawa K, Minami Y, Chung H, Suetomi Y, Onda H, Kitano M, Kawasaki T. Contrast advanced dynamic flow imaging and contrast pulse subtraction imaging: preliminary results in hepatic tumors. J Med Ultrasonics. 2002;29:195-204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Wen YL, Kudo M, Zheng RQ, Ding H, Zhou P, Minami Y, Chung H, Kitano M, Kawasaki T, Maekawa K. Characterization of hepatic tumors: value of contrast-enhanced coded phase-inversion harmonic angio. AJR Am J Roentgenol. 2004;182:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Yücel C, Ozdemir H, Gürel S, Ozer S, Araç M. Detection and differential diagnosis of hepatic masses using pulse inversion harmonic imaging during the liver-specific late phase of contrast enhancement with Levovist. J Clin Ultrasound. 2002;30:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Skjoldbye B, Pedersen MH, Struckmann J, Burcharth F, Larsen T. Improved detection and biopsy of solid liver lesions using pulse-inversion ultrasound scanning and contrast agent infusion. Ultrasound Med Biol. 2002;28:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Forsberg F, Piccoli CW, Liu JB, Rawool NM, Merton DA, Mitchell DG, Goldberg BB. Hepatic tumor detection: MR imaging and conventional US versus pulse-inversion harmonic US of NC100100 during its reticuloendothelial system-specific phase. Radiology. 2002;222:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Kim JH, Kim TK, Kim BS, Eun HW, Kim PN, Lee MG, Ha HK. Enhancement of hepatic hemangiomas with levovist on coded harmonic angiographic ultrasonography. J Ultrasound Med. 2002;21:141-148. [PubMed] |
| 15. | Kaul S. Myocardial contrast echocardiography. Curr Probl Cardiol. 1997;22:549-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Maresca G, Summaria V, Colagrande C, Manfredi R, Calliada F. New prospects for ultrasound contrast agents. Eur J Radiol. 1998;27 Suppl 2:S171-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Quaia E, Bertolotto M, Dalla Palma L. Characterization of liver hemangiomas with pulse inversion harmonic imaging. Eur Radiol. 2002;12:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | von Herbay A, Vogt C, Häussinger D. Late-phase pulse-inversion sonography using the contrast agent levovist: differentiation between benign and malignant focal lesions of the liver. AJR Am J Roentgenol. 2002;179:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Co-first-authors: Bao-Ming Luo and Yan-Ling Wen
Science Editor Guo SY Language Editor Elsevier HK