MATERIALS AND METHODS
Materials
Male SD rats, weighing (150 ± 5) g, were purchased from Beijing Vital River Company , corn oil from Carafour Supermarket, xanthan gum and maltose from Beijing Chemical Agent Company, edible alcohol from Beijing General Alcohol Brewing Company, carbonyl iron and pirazole from Sigma, USA, First antibody to MMPs 2, 3, 9, 13 and TIMPs 1, 2 was purchased from Antibody Diagnostic Inc, ADI, USA with the help of its Chinese Dealer, Shanghai Long Island Antibody Company. PV-6001 kits were from PowerVision, USA. ZLI-9030 and ZLI9001 were from Beijing Zhongshan Company.
Methods
Rats grouping: Normal (6), 4 d (8), 2 wk (8), 4 wk (10), 9 wk (12), 11 wk (12).
Modeling: The model of ALD was induced by intragastric infusion ( gavage) of a mixture of alcohol ( 5 g/d·kg), pirazole (30 mg/d·kg), corn oil (3 mL/d·kg), and carbonyl iron (35 mg/d·kg, decreased to 15 mg after 4 wk), a little xanthan gum and maltose, one time a day for 5 d consecutively, with 2 d off per week, until the end of the ninth wk, and all rats were maintained on a standard diet with water ad libitum.
Liver tissue management: The rats were executed at the end of 4 d, 2 wk, 4 wk, 9 wk and 11 wk, respectively. Harvested livers were split and fixed for electron microscopy, hematoxylin and eosin and Masson complex staining, and a portion was snap frozen for biochemical and molecular analysis. Further sections were cut from each liver, deparaffinized, HE and Masson staining. Histological analysis of each liver was undertaken, then the sections subjected to amylopsin antigen retrieval before stained with the two-step method for antigen MMPs 2, 3, 9, 13 and TIMPs 1 and 2. The semi-quantitive computations were conducted on the image analyzing system MIS-2000 from 3Y Company, USA, The activity of MMP-2 and MMP-9, Western blotting of TIMPs 1 and 2 were undertaken with Bio-Rad Electrophoresis System, pictures were taken and analyzed with Image master VDS from Pharmacia Biotech Company.
Statistical analysis
All values were expressed as mean ± SD. ANOVA was used to determine the significance of differences among the six groups. P < 0.05 was considered statistically significant.
RESULTS
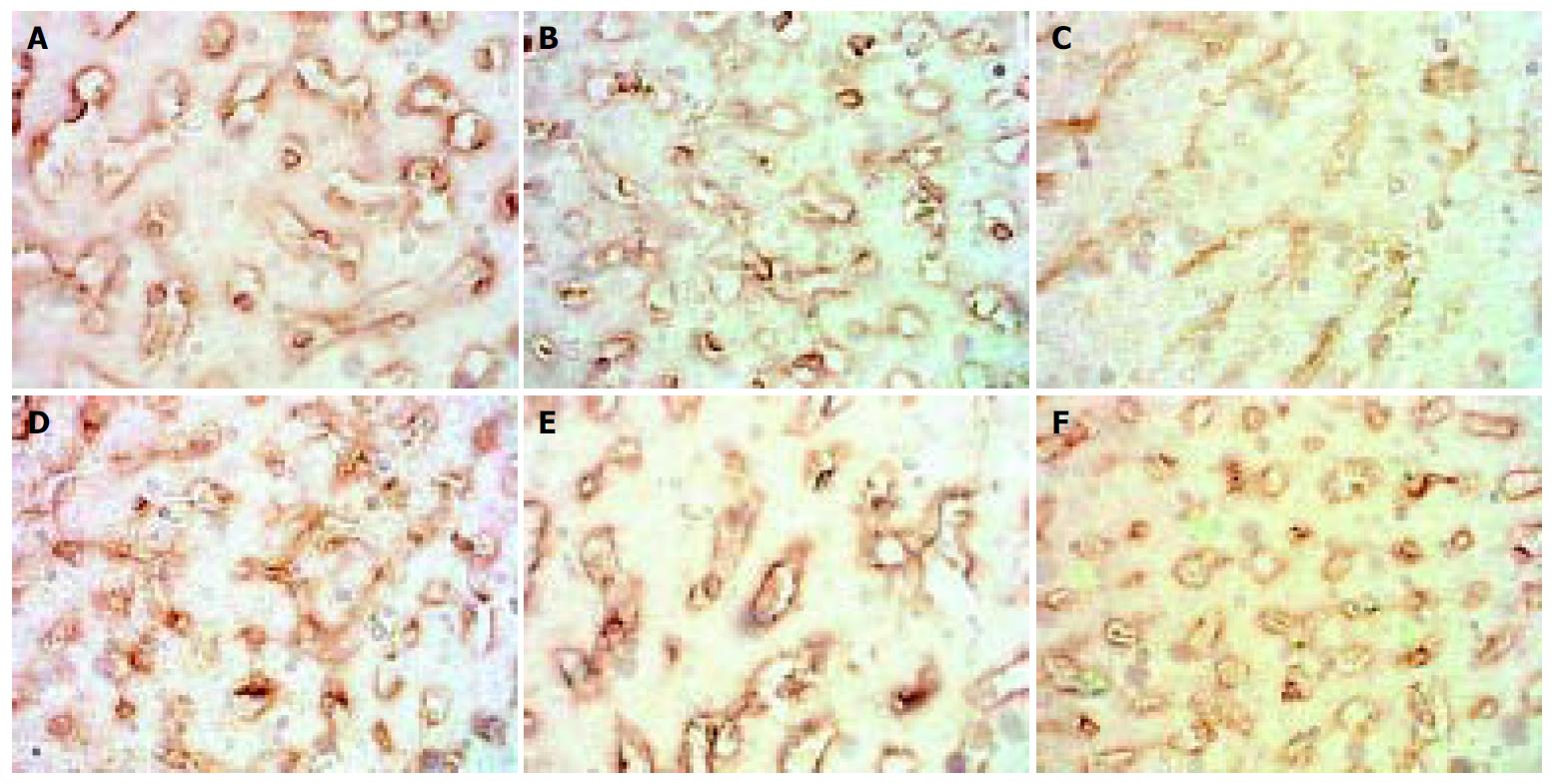
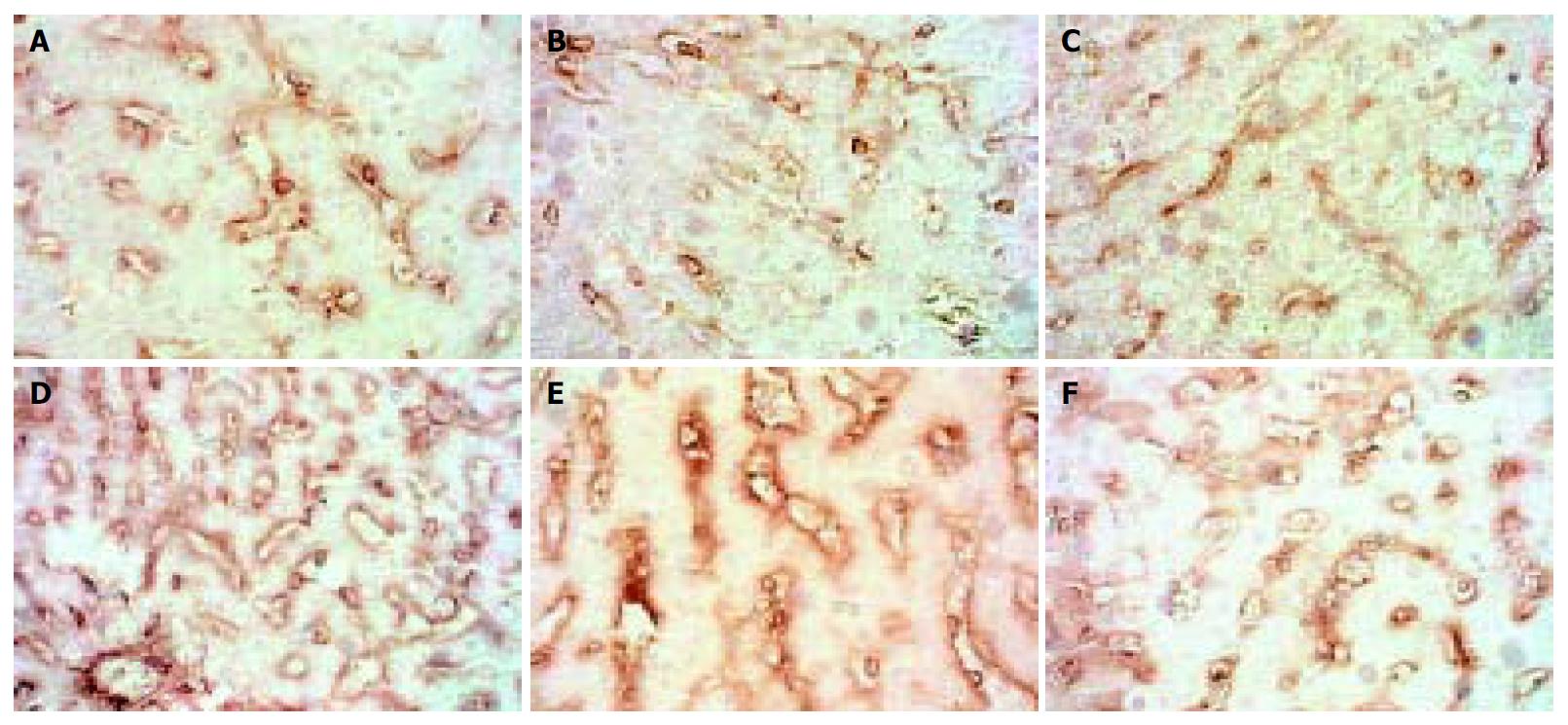
The expression of MMP-2 in normal rats was at moderate level. However, during modeling it showed reducing curve changes and without recovery even after modeling was stopped for 2 wk. (Figure 1).
Figure 1 Dynamic changes of MMP-2.
A: Normal rat, × 400 B: 4 d group, × 400 C: 2 wk group, × 400 D: 4 wk group, × 400. E: 9 wk group, × 400 F: 11 wk group, × 400.
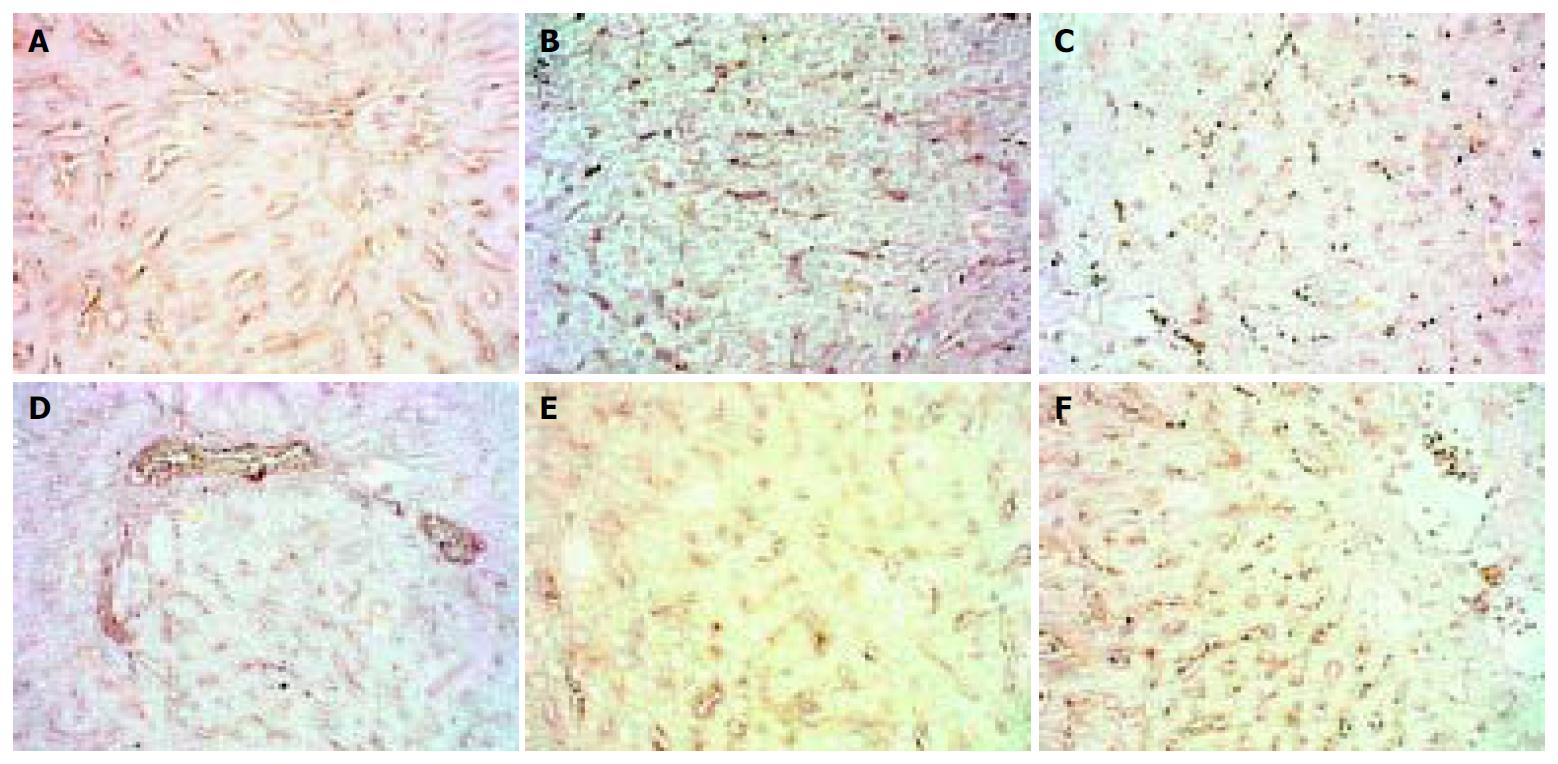
The expression of MMP-3 showed a similar pattern to that of MMP-2 but weaker, when modeling was stopped it recovered to normal level. (Figure 2).
Figure 2 Dynamic changes of MMP-3.
A: Normal rat, × 200 B: 4 d group, × 200 C: 2 wk group, × 200 D: 4 wk group, × 200. E: 9 wk group, × 200 F: 11 wk group, × 200.
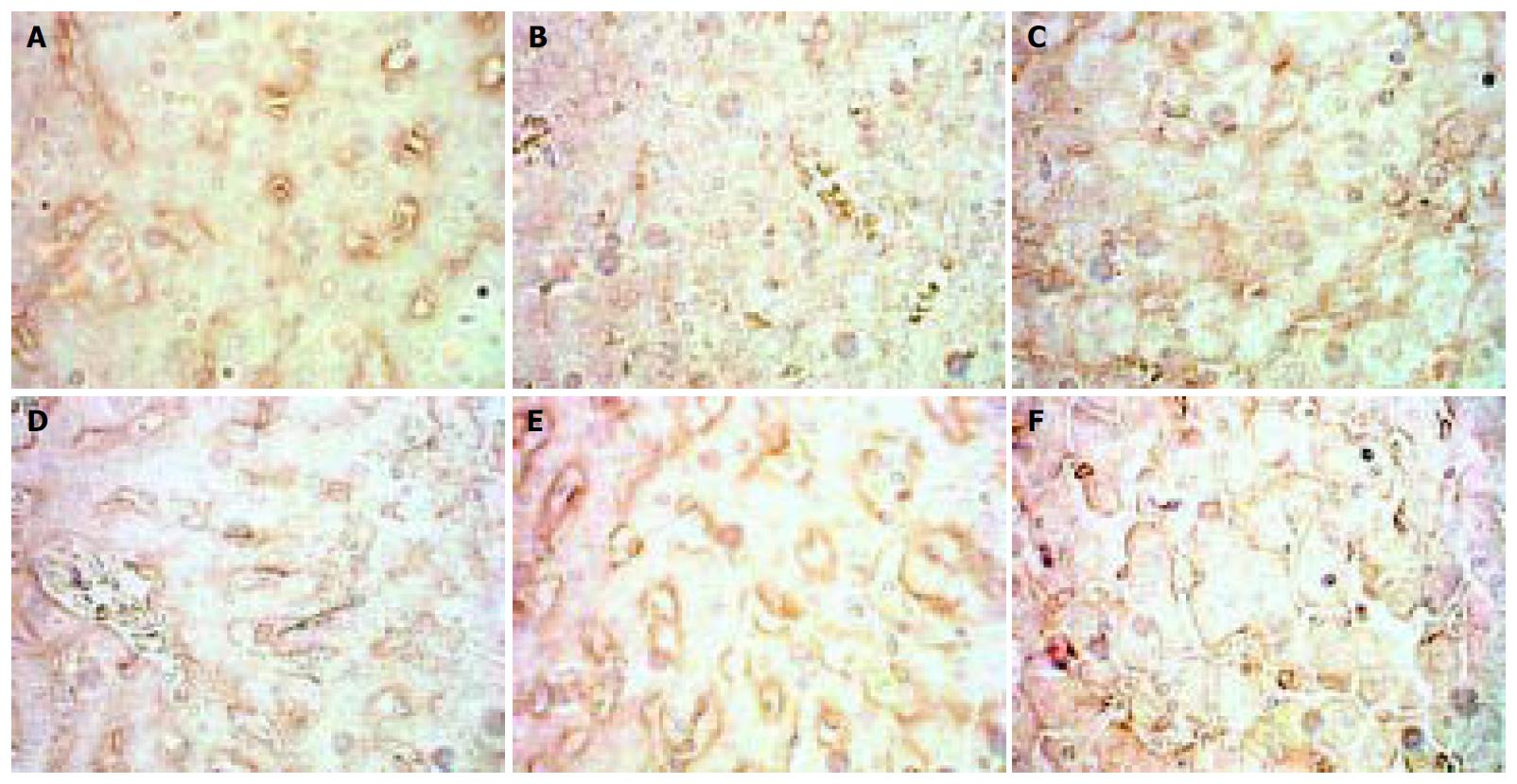
The situation of MMP-9 was different from that of the two above. MMP-9 decreased in the 4 d and 2 wk groups, but in the 4 wk group and the following MMP-P showed higher expression than that of the normal rats with no significance. (Figure 3).
Figure 3 Dynamic changes of MMP-9.
A: Normal rat, × 400 B: 4 d group, × 400 C: 2 wk group, × 400 D: 4 wk group, × 400 E: 9 wk group, × 400 F: 11 wk group, × 400.
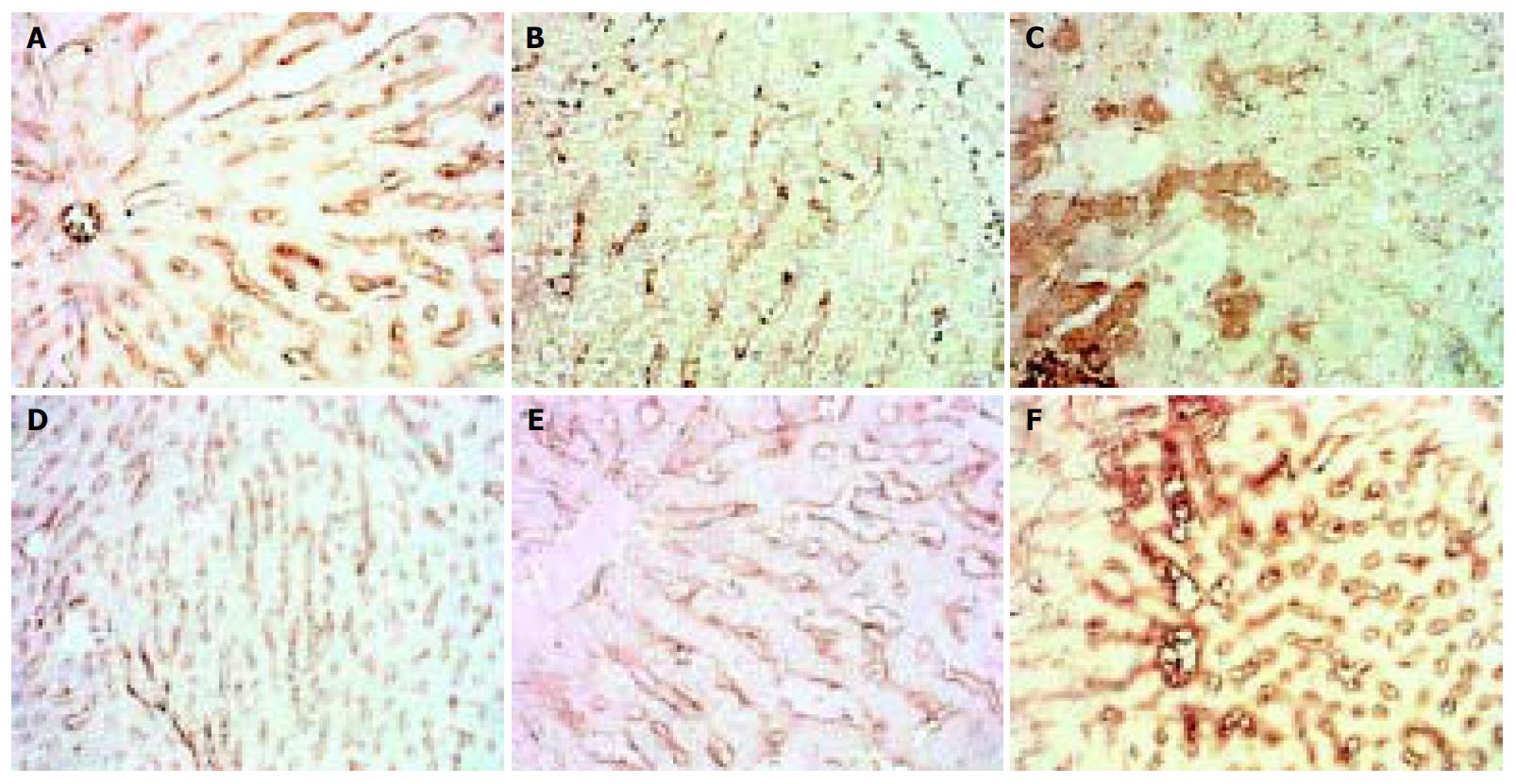
The expression of MMP-13 showed reduction in the 4 d and 2 wk groups, but recovered in the 4 wk group and then sustained, when modeling was stopped it showed higher expression than normal. (Figure 4).
Figure 4 Dynamic changes of MMP-13.
A: Normal rat, × 200 B: 4 d group, × 200 C: 2 wk group, × 200 D: 4 wk group, × 200 E: 9 wk group, × 200 F: 11 wk group, × 200.
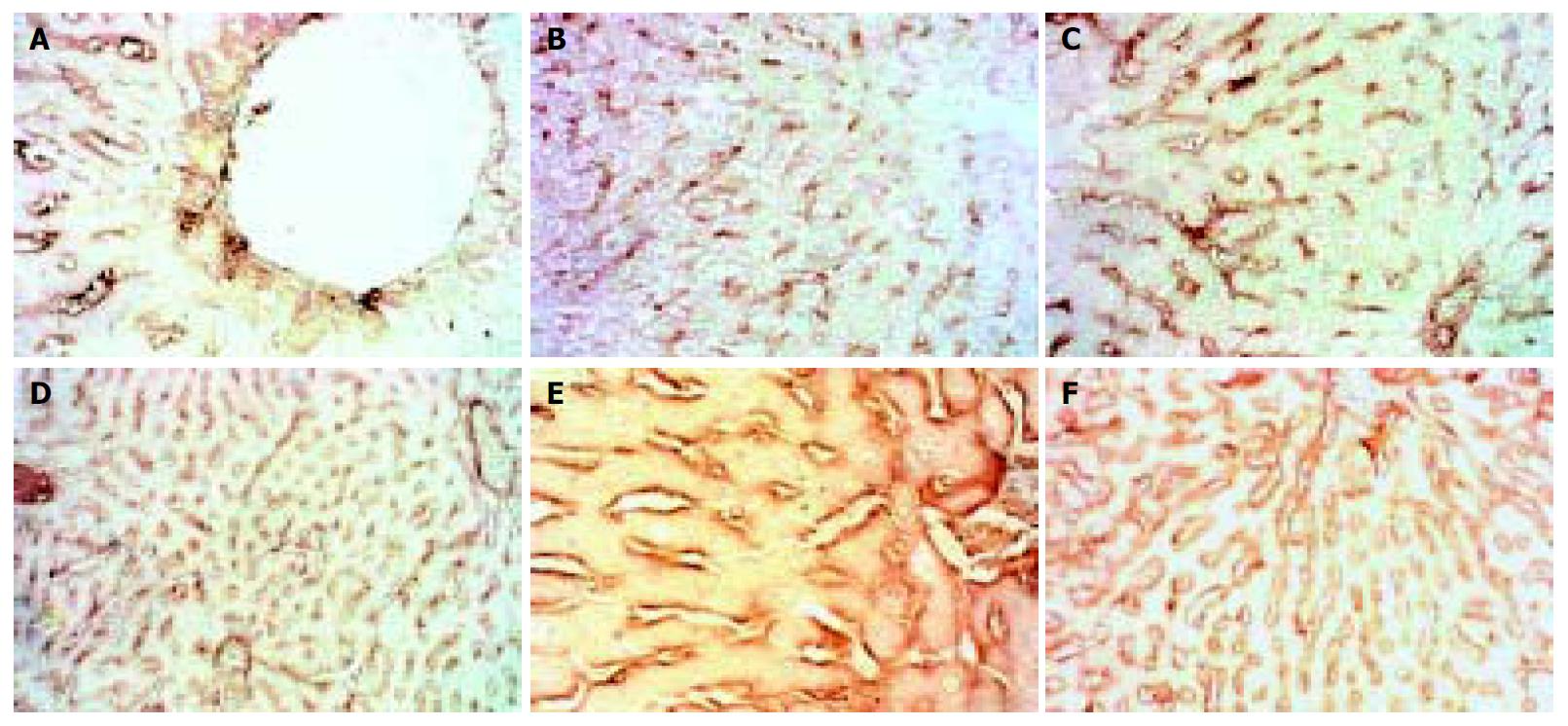
The expression of TIMP-1 in the 4 d and 2 wk groups showed reduction, after then it gave distinct higher expression and did not recover to normal level even after modeling was stopped for 2 wk. (Figure 5).
Figure 5 Dynamic changes of TIMP-1.
A: Normal rat, × 400 B: 4 d group, × 400 C: 2 wk group, × 400 D: 4 wk group, × 400 E: 9 wk group, × 400 F: 11 wk group, × 400.
The expression of TIMP-2 showed different type from that of TIMP-1. In normal rats it had a high level with some hepatocytes expressing, but as soon as modeling began it fell rapidly, then gradually ascended as modeling went on, but after modeling was stopped for 2 wk it was still lower than normal. (Figure 6).
Figure 6 Dynamic changes of TIMMP-2.
A: Normal rat, × 200 B: 4 d group, × 100 C: 2 wk group, × 200 D: 4 wk group, × 100 E: 9 wk group, × 400 F: 11 wk group, × 100.
We failed in this model to find any activities of MMP-2 and MMP-9 with zymography.
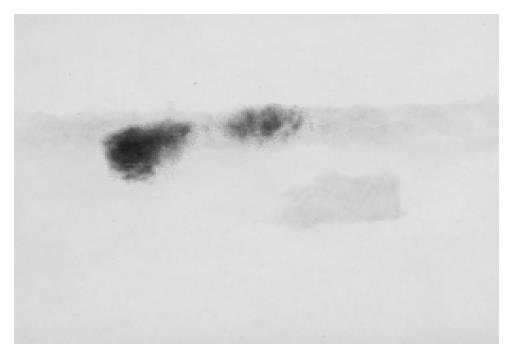
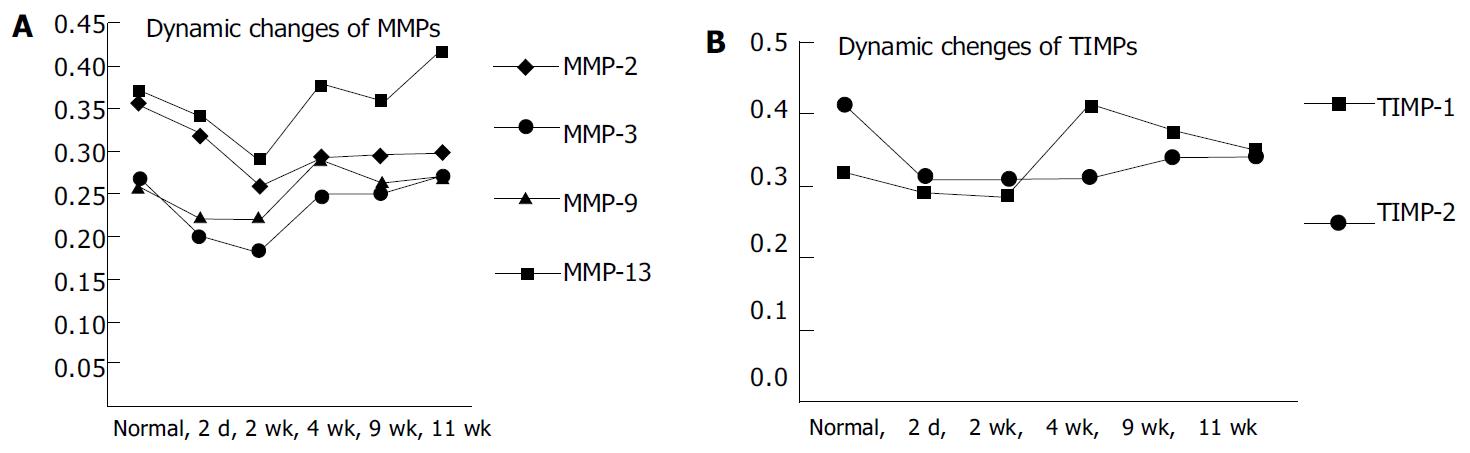
Western blotting tests showed that in the 4 wk and 9 wk groups there were strong expressions of TIMP-1, but in other groups there were not. As to TIMP-2 we did not find its expression. (Figure 7, Figure 8).
Figure 7 Expression of TIMP-1 in the 4 wk and 9 wk groups.
Figure 8 Dynamic changes of MMPs 2, 3, 9, 13 and TIMPs 1, 2 A: Dynamic changes of MMPs 2, 3, 9, 13.
B: Dynamic changes of TIMPs 1, 2.
DISCUSSION
China’s prosperity is accompanied with cases of alcoholic liver disease growing day by day. Alcohol can stimulate hepatic stellate cells (HSCs) to proliferate and produce various fibrosis cytokines, which in turn result in hepatic fibrosis in the end. Abstinence itself can not reverse hepatic fibrosis, and more over, temperance is usually very difficult. So it is important to probe into the mechanism of hepatic fibrosis reversion in ALD research. Most of the recent reports[1-11] reveal that the formation and prognosis of hepatic fibrosis were all related with MMPs/TIMPs, and this viewpoint has been accepted as a common sense. So we probed into the important factors among MMPs/TIMPs, and respective discussions were as follows.
Matrix Metallopretinase-2 (MMP-2) is an important factor in the metabolism of type IV collagen in sinusoid basement. It has a peculiar activation course taking place on the membrane and forms a triple complex bridged with MT1-MMP by TIMP-2. Deactivation could be triggered by more competitive TIMP-2 to conjugate with MMP-2 to restrain the formation of triple complex. Because of local expression of TIMP-2 it is hypothesized that MMP-2 could not be activated on a long trip, and it is only transient and local. Activated MMP-2 could decompose type IV collagen in the basement, resulting in broken micro circumstances of HSC and promoting HSC to activate, proliferate and migrate. Activated MMP-2 also could play an important role in reversion of hepatic fibrosis, metastasis of tumors, MOF induced by endotoxin, apoptosis of HSC, etc[12-26]. MMP-2 is mainly composed and secreted by HSC in liver. Activation induced by various pathogens might need different time, and strong stimulation would result in rapid activation[27]. It was reported that[28] during reversion period activated MMP-2 was in favor of fibrosis reversion, so it was thought that MMP-2 played another role at this time because activated MMP-2 could activate HSC and promote fibrosis[29,30].
The results of our experiment were different from those of others. MMP-2 showed moderate expression in SD rat liver slides along the sinus in normal, began to fall during modeling, instead of rising up as reported, meanwhile zymograghy tests did not show any activity of it. So we thought that it was peculiar that lowly expressed MMP-2 could also break down type IV collagen in sinus basement markedly. Capillarization of this model was not typical because of lower contents of type IV collagen than normal. This might imply lowly expressed MMP-2 not only decomposed parts of type IV collagen in sinus basement but also obstructed its accumulation, resulting in atypical capillarization in the end. It could not achieve success unless TIMP-2 markedly decreased its expression so as to alleviate MMP-2, and our experiment showed an authentic result just like this. This phenomenon may exclude the possibility that only high activity of MMP-2 could result in breakage of sinus basement and progression of fibrosis, and it also may imply that MMP-2 is not the most important factor imposed on the metabolism of type IV collagen in sinus basement. Our results also showed that the cooperation of MMP-2/TIMP-2 could make the contents of type IV collagen in sinus basement become normal after the modeling was stopped for 2 wk. This was not consistent with other scholar’s report[28] that apoptosis of HSC could decrease the expression of TIMP-2 and promote the activity of MMP-2. The differences between species, modeling, stages and types of pathology may be related with these diversities. The fast and accurate adjusting mechanism of MMP-2’s function influenced heavily by TIMP-2, rather than TIMP-1, might be a commonfeature of different models.
MMP-3 also called stromelysin 1, originates from almost the same cells as MMP-1 but does not show synchronization in expression. MMP-3 has a wide range of substrates but important one may be laminin. it can also activate MMP-1, but there are not many reports about it. It has been reported that the measurement of serum MMP-3 was of little use for assessing fibrolysis in chronically diseased livers. However, because of the distinct pathological changes in sinusoid and peri-sinusoid in alcoholic liver disease, the value of MMP-3 rose to some extent. We found that accumulation of laminin in basement was quite prominent during capillarization. It was evident that the sustained low expression during modeling of MMP-3 would be in favor of laminin’s accumulation. The accumulated laminin in basement was still high after the modeling was stopped for 2 wk in spite of the normal expression of MMP-3, which might imply not only that the strong inhibiting effect of TIMP-1 on MMP-3 could result in delayed fashion of metabolism of laminin compared to that of type IV collagen, but also that sinusoid pathological changes were not entirely modulated by MMP-2/TIMP-2, and this is important.
MMP-9 is also called gelatinase B, the majority of which is secreted by kupffer cells in liver. MMP-9 could also decompose type IV collagen in basement, and this might happen a little earlier than that of MMP-2, implying MMP-9 might take part in the early events of fibrosis. In this model, however, MMP-9 had lower expression than MMP-2, and continued after the modeling began. It could be thought that MMP-9 did not play role in this model entirely. It was reported[31] that widespread capillarization in cirrhosis suggested no MMP-2 activity but low MMP-9 activity, implying potent importance of MMP-9 in reversion of fibrosis. In this model in the 4 wk and 11 wk groups MMP-9 showed little higher expression than normal but still lower than that of MMP-2, actually it might not affect the accumulation of type IV collagen, so it was not an important factor. This result did not show any consistency with other reports[29,30].
MMP-1, an interstitial collagenase (in rats it is MMP-13) is the most important enzyme secreted by kupffer cells and HSC in liver, and after secretion it is activated and the course may be related to plasminogen activator system. MMP-1/MMP-13 could be deactivated by forming a complex with TIMP-1 at a ratio 1:1. It was reported that kupffer cells in liver were one of the main originations of MMPs, including MMP-13. It was also reported recently that[32] the rise of activity of MMP-13 would significantly improve cirrhosis, which could be realized by stimulating kupffer cells. This implied another way to reverse fibrosis. The activity of MMP-13 was also needed to ensure normal metabolism of hepatocytes[33-35]. We found in our experiment that MMP-13 expressed themselves moderately mainly along sinusoid in normal rats which would support such a kind of viewpoints. MMP-13 decreased to some extent when the modeling began, in the 2 wk group some hepatocytes and kupffer cells showed active expression of MMP-13, then the expression along sinusoid again showed an ascending trend. In the 4 wk group when fibrosis was most severe the expression of MMP-13 was almost the same as normal, meaning that MMP-13 itself could not affect the course of peri-sinusoidal fibrosis. In the reversion phase the expression of MMP-13 was significantly higher than normal and the active stains implied that kupffer cells might restart their expression, which would be helpful to fibrosis reversion, but the accumulation of type I collagen was not affected and became more severe with the modeling, evidently the inhibiting effect of TIMP-1 on MMP-13 should be the cause. The higher expression of MMP-13 in the reversion phase actually could not help reverse peri-sinusoidal fibrosis, demonstrating once more that TIMP-1 was the most important factor during hepatic fibrosis.
TIMP-1 is produced by kupffer cells, HSC and myofibroblasts in liver, but most of it is mainly produced by activated HSC, It could be induced by TGF beta, interferon or pentoxifylline. TIMP-1 could inhibit most of MMPs except MMP-14 and MMP-19 by integrating them at a ratio 1:1 to form a complex. Injured liver tissues showed more expression of TIMP-1, and in turn, this would help interstitial fibrils to accumulate[33-36]. Serum TIMP-1 had a close relation to hepatic pathology, serum TIMP-1 could forecast hepatic pathological changes and prognosis, and could also be used as an index to evaluate the treatment. The transgenic model lacking TIMP-1 gene and anti-oligonucleotide treatment model aiming at TIMP-1 were established to find that without injury TIMP-1 itself could not result in fibrosis, but if there were injuries TIMP-1 would significantly accelerate the course in which fibrosis was formed. This might imply omni directional and all target treatment for hepatic fibrosis should be considered, nevertheless targeted and usual treatment still took TIMP-1 as a comprehensive index for evaluation[37-41]. Overexpression of TIMP-1 could keep hepatic fibrosis by holding back the apoptosis of HSC, otherwise the down-regulation of TIMP-1 would promote MMPs to be activated, and in turn, fibrils decomposed, HSC apoptosis took place and fibrosis reversed. All this emphasized the importance of TIMP-1.
We found in this model that at the early phase TIMP-1 showed a relatively low expression, in the 4 wk and 9 wk groups TIMP-1 showed evident expression mainly along sinusoid, which might be attributed to the widely activated HSCs. The over-expression of TIMP-1 would inhibit MMP-13 and MMP-3, resulting in accumulation of type I collagen and laminin, which in turn would be in favor of the formation of capillarization and peri-fibrosis. This discovery supported the importance of TIMP-1. It was also underpinned by Western blotting tests which showed TIMP-1 expressed itself most evidently in the 4 wk and 9 wk groups. In this model after the stimulus was ceased for 2 wk TIMP-1 still showed high expression, which might imply that activated HSCs were difficult to start spontaneous apoptosis, and in turn, TIMP-1 would not cease its expression by itself, which again showed it was a key step to reduce the expression of TIMP-1 in order to reverse fibrosis. Our results demonstrated that even if MMP-13 expressed highly it still would be inhibited by TIMP-1 to result in accumulation of type I collagen. It was reasonable that normal-level MMP-3 was inhabited by over-expressed TIMP-1, resulting in high content of laminin in basement of sinusoid, and capillarization and peri-sunusoidal fibrosis were not drastically improved. So this should be attributed to the high expression of TIMP-1.
The ability of TIMP-2 to integrate MMP-2 is 7-9 fold higher than that of TIMP-1, so if it is true it is the most important factor imposed on the metabolism of type IV collagen in basement of sinus. MMP-2 itself could not affect the course of capillarization as found in this experiment. We also found in normal SD rat livers that TIMP-2 was highly expressed in hepatocytes and kupffer cells, but transformed to HSCs with few active stains along sinusoid as the modeling began, which might imply activated HSCs would be the main origin of TIMP-2. During the modeling decreased expression of TIMP-2 might weaken the inhibition on MMP-2 and result in the decomposition of type IV collagen in basement and could not accumulate to help form typical capillarization. This was a typical feature of our model. After the modeling was stopped for 2 wk, the expression of TIMP-2 ascended but was little lower than normal, and the content of type IV collagen returned to normal level, which implied the gradually rising TIMP-2 could show its suppressing function on MMP-2, so that type IV collagen was protected and became normal. This demonstrated the significant effect of TIMP-2 and MMP-2 on the metabolism of type IV collagen in basement. However, TIMP-2 was apparently the more important.
In conclusion, the pathological changes of ALD model with fibrosis grades I-II can be controlled by TIMPs, especially the expression of TIMP-1 and TIMP-2 has significant effects on the prognosis of capillarization and peri-sinusoidal fibrosis, and MMPs are always dominated by TIMP-1 and TIMP-2. So the fatal step to reverse fibrosis might be to block or modulate the expression of TIMP-1 and TIMP-2.