Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3628
Revised: March 6, 2004
Accepted: March 13, 2004
Published online: December 15, 2004
AIM: To evaluate the diagnostic sensitivity and accuracy and the cost-effectiveness of this technique in the detection of gastroenteropancreatic carcinoid tumors and their metastases in comparison with conventional imaging methods.
METHODS: Somatostatin receptor scintigraphy (SRS) was performed in 24 patients with confirmed carcinoids and 7 under investigation. The results were compared with those of conventional imaging methods (chest X-ray, upper abdominal ultrasound, chest CT, upper and lower abdominal CT). Also a cost-effectiveness analysis was performed comparing the cost in Euro of several combinations of SRS with conventional imaging modalities.
RESULTS: SRS visualized primary or metastatic sites in 71.0% of cases and 61.3% of conventional imagings. The diagnostic sensitivity of the method was higher in patients with suspected lesions (85.7% vs 57.1%). SRS was less sensitive in the detection of metastatic sites (78.9% vs 84.2%). The undetectable lesions by SRS metastatic sites were all in the liver. Between several imaging combinations, the combinations of chest X-ray/upper abdominal CT/SRS and chest CT/upper abdominal CT/SRS showed the highest sensitivity (88.75%) in terms of the number of detected lesions. The combinations of chest X-ray/upper abdominal US/SRS and chest CT/upper abdominal ultrasound/SRS yielded also a quite similar sensitivity (82%). Compared to the cost of the four sensitive combinations the combination of chest X-ray/upper abdominal ultrasound/SRS presented the lower cost, 1183.99 Euro vs 1251.75 Euro for chest CT/upper abdominal ultrasound/SRS, 1294.93 Euro for chest X/ray/upper abdominal CT/SRS and 1362.75 Euro for chest CT/upper abdominal CT/SRS.
CONCLUSION: SRS imaging is a very sensitive method for the detection of gastroenteropancreatic carcinoids but is less sensitive than ultrasound and CT in the detection of liver metastases. Between several imaging combinations, the combination of chest X-ray/upper abdominal CT/SRS shows the highest sensitivity with a cost of 1294.93 Euro.
- Citation: Dimitroulopoulos D, Xynopoulos D, Tsamakidis K, Paraskevas E, Zisimopoulos A, Andriotis E, Fotopoulou E, Kontis M, Paraskevas I. Scintigraphic detection of carcinoid tumors with a cost effectiveness analysis. World J Gastroenterol 2004; 10(24): 3628-3633
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3628
The carcinoid tumor, argentaffinoma, is a member of a very exclusive neoplastic family known as neuroendocrine or amine precursor uptake and decarboxylation (APUD) tumors.
Carcinoid tumor has been found to arise from almost every organ and system derived from the primitive entoderm, but most frequently originated from the gastrointestinal (GI) tract, accounting for approximately half of all GI endocrine tumors[1].
Over 95 per cent of all GI carcinoids are located in only three sites: the appendix, rectum and small intestine.
Irrespectively to their location, carcinoids are capable of producing one or more of the following substances: 5-hydroxy-tryptamine (serotonin), gastrin kinin-peptide, histamine, catecholamine and glucagon. Some of them induce systemic manifestations known as the carcinoid syndrome characterized by flushing, diarrhea, right-sided heart disease and wheezing[2,3].
Carcinoid tumors are rare (incidence: about 2/100000 people)[4], malignancy-that is mainly liver metastases, may be encountered in 10%-60% of cases depending on the site of the primary tumor[5,6]. Metastases are observed in less than 2% of carcinoids 1 cm or less in size. In contrast, nearly all carcinoids 2 cm or greater show evidence of metastatic spread[1].
Tumor localization is essential since surgery remains the optimal treatment for most patients without metastases[7,8].
Curative surgery is difficult since primary tumors are frequently very small (< 1 cm) and potentially undetectable by conventional imaging. When liver metastases occur, staging of these patients is essential for therapeutic manipulation.
Tumor localization for accurate staging and therapeutic management justifies the use of sophisticated imaging techniques such as somatostatin receptor scintigraphy (SRS)[9,10].
Since the introduction of somatostatin receptor imaging in 1989[9], many reports on the usefulness and limitation of this technique have been published.
It has been shown by autoradiography using 125I-labeled octreotide that endocrine tumors of GI tract and especially carcinoids possess somatostatin receptors[11-13]. When octreotide is labeled with radionuclides such as 123I[14,15] or 111In, the specific receptor binding can be exploited for the scintigraphic in vivo demonstration of receptor-expressing tumors[9,10,16].
The radiolabeled analogue 111In-DTPA-octreotide also known as octreoscan is cleared by renal than hepatobiliary route, thus causing less artifacts on hepatic and mesenteric imaging[17,18].
A total of 31 patients (18 males, 13 females, age ranged 27-73 years) under SRS 111In-pentatreotide were enrolled between April 1997 and October 2003 at “Agios Savvas” Cancer Hospital (Section of Nuclear Medicine), Athens, Greece. Their data are listed in Table 1.
| Pt | Sex | Age (yr) | Primary tumor site | Metastases | Carcinoid syndrome related signs and symptoms | SRS primary metastases sites | Conventional imaging methods primary metastases sites | ||
| Patients with confirmed tumors | |||||||||
| 1 | F | 69 | Stomach | - | - | - | - | ||
| 2 | M | 58 | Stomach | + | - | - | - | ||
| 3 | M | 55 | Duodenum | - | - | - | - | ||
| 4 | M | 55 | Small intestine | Liver | + | + | - | + | |
| 5 | F | 69 | Small intestine | + | - | - | - | ||
| 6 | M | 33 | Appendix | Lymph nodes | + | + | - | - | |
| 7 | F | 27 | Appendix | - | - | - | - | ||
| 8 | M | 39 | Appendix | Liver-ribs | Diarrhoea | + | + | + | + |
| 9 | M | 59 | Caecum | - | - | + | - | ||
| 10 | F | 64 | Caecum | + | - | - | - | ||
| 11 | M | 69 | Rectum | + | - | - | - | ||
| 12 | F | 57 | Rectum | + | - | - | - | ||
| 13 | F | 49 | Pancreas | Liver-lung | Diarrhoea | + | + | + | + |
| 14 | M | 58 | Pancreas | Liver-lymph nodes | Diarrhoea flushes | + | + | + | + |
| Patients previously operated | |||||||||
| 15 | F | 42 | Stomach | Liver | + | + | |||
| 16 | M | 34 | Appendix | Liver | - | + | |||
| 17 | M | 36 | Appendix | Lymph nodes | + | + | |||
| 18 | F | 40 | Appendix | Liver-Lymph nodes | + | + | |||
| 19 | M | 69 | Small intestine | Lymph nodes | + | + | |||
| 20 | M | 61 | Small intestine | Liver | + | + | |||
| 21 | F | 67 | Caecum | Lymph nodes | + | + | |||
| 22 | F | 56 | Colon Liver | - | + | ||||
| 23 | M | 72 | Rectum | Liver-lymph nodes | - | + | |||
| 24 | M | 59 | Pancreas | Liver | - | + | |||
| Patients with suspected carcinoid tumors | |||||||||
| 25 | M | 34 | Appendix | Flushes | - | - | - | - | |
| 26 | F | 33 | Appendix | Diarrhoea | + | - | - | - | |
| 27 | M | 51 | Small intestine | Liver | Diarrhoea flushes | - | + | - | - |
| 28 | M | 62 | Caecum | Diarrhoea | + | - | + | - | |
| 29 | M | 61 | Caecum | Liver-lymph nodes | Diarrhoe flushes | + | + | + | - |
| 30 | F | 71 | Caecum | Liver | Diarrhoea flushes | + | + | + | + |
| 31 | F | 73 | Pancreas | Liver-lungs | Diarrhoea flushes | + | + | + | + |
Inclusion criteria required histological or cytological confirmation of a presently or previously operated abdominal carcinoid, or for patients with suspected tumors, a history of carcinoid syndrome-related signs and symptoms with an additional elevation of urinary 5-HIAA. All patients gave informed consent to participation in the study, which was approved by the ethics committee of our hospital.
Seven of the patients were under investigation for suspected carcinoids in different sites (caecum, appendix, small intestine, pancreas) while the remaining 24 had histologically/cytologically confirmed tumors, in 10 of them the primary lesion was excised. All gastric carcinoids were type II or “mixed cellular composition” gastric carcinoid tumors.
Seven patients were treated by octreotide prior to SRS, in all but 3 of them therapy was withdrawn 36 h prior to somatostatin receptor imaging in order to lift the blockade of SRS. In the rest 3 patients the 3-d withdrawal period was clinically impossible. The administration dose of octreotide in these patients was 0.5 mg daily.
A low-residue diet was started 3 d prior to SRS and stopped at the end of the imaging procedure. Twelve hours before the injection of the tracer a mild laxative was administered to minimize the false positive results, because a small quantity of the administrated dose underwent hepatobiliary excretion.
Patients were well hydrated prior to radioactive drug administration to increase renal clearance and to reduce radiation uptake to the thyroid, kidneys, bladder and other target organs. All individuals in our study had normal thyroid and renal function.
111In-pentetreotide (Octreoscan, Mallinckrodt Medical BV, Petten, Holland) was supplied as two vial kits. The first contained 111In as 111InCl3 diluted in 1.1 mL hydrochlorid acid and the other lyophilized pentetreotide. After reconstitution the pH of the final product was between 3.8 and 5. This product might be diluted with normal saline solution because the dilution would raise the pH slightly.
After an incubation period of 30 min at room temperature and before the administration, instant thin layer chromatography (ITLC) for quality control was performed. The dose for a planar investigation was 111 MBq (3.3 mCi) of octreoscan.
The radiolabeled somatostatin analogue was administered as an intravenous bolus and no side effects were observed after i.v. injection.
Imaging Whole body scanning and planar images were obtained with a large field of view gamma camera (Siemens) equipped with a medium- energy, parallel-hole collimator. The pulse-height analyser windows were centered over both 111In peaks (172 keV and 245 keV) with a window width of 20%. Data from both windows were added to the acquisition frames. Images were obtained 24 and sometimes 48 h after tracer administration.
Scintigraphic results were compared with those obtained by other imaging methods such as chest X-ray performed, upper abdominal ultrasonography, chest CT scan performed, upper and lower abdominal CT scans.
Magnetic resonance imaging of the abdomen and digital abdominal angiography was performed in few cases, but because of the small number of patients these imaging techniques were not taken into account.
Statistical comparison between SRS and conventional imaging methods for the detection of primary and metastatic sites, globally and in each group of patients, was performed using McNemar’s test based on discordant pairs. A P value ≤ 0.05 was considered statistically significant.
A cost-effectiveness analysis was performed comparing the SRS with the conventional imaging methods (chest X-ray, upper abdominal ultrasonography, chest CT scan and upper and lower abdominal CT scan) in several combinations. The cost of each diagnostic procedure was calculated.
The personnel cost was calculated as the cost of a working hour for each person (physician, technician, nurse, assistant personnel).
The cost of materials was calculated including radiographs, injection systems, contrast liquids and kit material.
The equipment cost was calculated in working hours. A yearly payment on an annuity basis at 8% and a term of 5 and 8 years were used to calculate the cost of ultrasound equipment, scanners and gamma cameras. The prices of equipment were those of 2000. Maintenance cost was estimated at 8% of new value.
The housing and overhead costs based on the number of square meters required to investigate a patient, including the cost of furniture, cleaning, telephone and services of various overhead departments.
Costs were built up from a database of health care cost elements in Greece and from the currently applicable prices of octreoscan and contrast material.
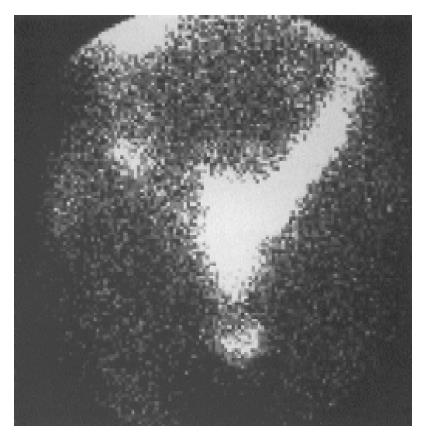
SRS imaging visualized the primary tumor or metastatic sites in 22 (71.0%) out of 31 patients who had histologically-cytologically confirmed carcinoid tumors or were under investigation for highly suspected carcinoid (16/24-66.7% and 6/7-85.7% respectively) (Figure 1).
Conventional imaging was positive in 19 (61.3%) patients (4/7- 57.1% with suspected carcinoids and 15/24-62.5% with known tumors). Thus, SRS provided additional detection sites compared with conventional imaging methods even if the global detection rate (71.0% vs 61.3%) was quite similar. Detection of primary sites was 33.3% higher with SRS than with conventional methods (71.4% vs 38.1% respectively, P = 0.039). The primary lesions were detected by SRS in 15 (71.4%) of 21 patients. Octreoscan scintigraphy failed to detect primary tumors in 6 patients (28.6%), 4 with known lesions (stomach, duodenum, appendix, caecum) and 2 under investigation (appendix, small intestine).
The 6 lesions (≤ 0.7 cm) that were not visualized after injection of 111In-pentetreotide were detected by endoscopy (3) or surgery (3) and diagnosed by histology. Only 1 out of 6 lesions was visualized by conventional imaging methods (patient No 9). Further analysis of the results from each group of patients with residual primary tumors did not reveal any statistically significant difference between the two methods (P > 0.05).
The positive detection rate in metastatic sites was similar by SRS and conventional imaging methods, which was 48.4% and 51.6% respectively (P > 0.05).
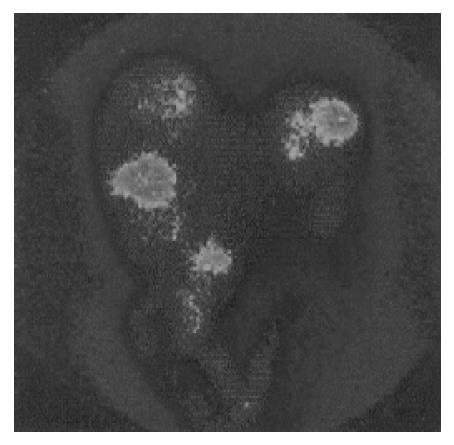
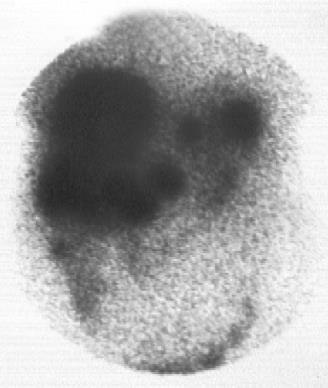
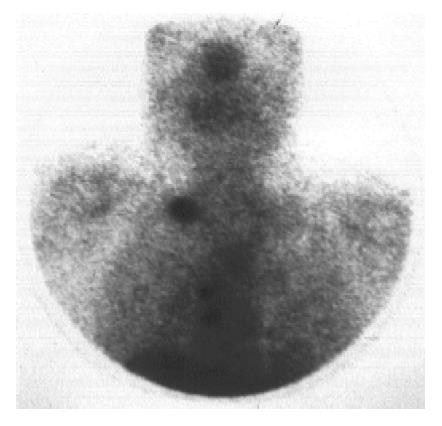
In the 19 patients with metastatic disease, SRS detected metastatic lesions in 15 cases (78.9%) (Figure 2, Figure 3, Figure 4, Figure 5) and failed to visualize metastatic sites in 4 patients (21.1%), all in liver were subsequently detectable by ultrasonography and CT scans. On the other hand, conventional imaging visualized metastases in 16 (84.2%) patients with a detection rate of 5.3%, higher than that of SRS.
False negative results of SRS and conventional imaging methods for primary and metastatic tumor sites in patients with known and suspected carcinoids are shown in Table 2, Table 3.
| Method | Detection of carcinoids | Patient No | False-negative (%) | |
| SRS | Known carcinoids | 4/14 patients | 1, 3, 7, 9 | 28.57 |
| Suspected carcinoids | 2/7 patients | 25, 27 | 28.57 | |
| Conventional | Known carcinoids | 10/14 patients | 1-7, 10-12 | 71.42 |
| imaging methods | Suspected carcinoids | 3/7 patients | 25, 26, 27 | 42.85 |
| Method | Detection of carcinoids | Patient No | False-negative (%) | |
| SRS | Known carcinoids | 4/24 patients | 16, 22-24 | 16.66 |
| Suspected carcinoids | 0/7 patients | - | 0 | |
| Conventional | Known carcinoids | 1/24 patients | 6 | 4.16 |
| imaging methods | Suspected carcinoids | 2/7 patients | 27, 29 | 28.6 |
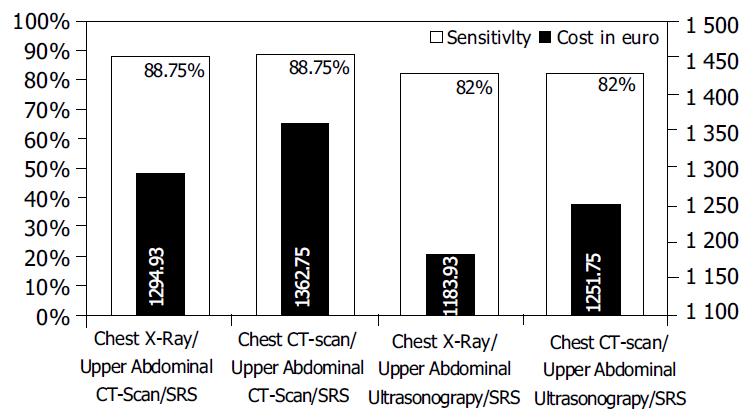
Comparison of 8 imaging combinations showed that the combinations of chest X-ray/upper abdominal CT scan/SRS and chest CT scan/upper abdominal CT scan/SRS achieved the highest sensitivity in the detection of primary and metastatic lesions (88.75% for each one).
The combinations of chest X-ray/upper abdominal ultrason-ography/SRS and chest CT/upper abdominal ultrasonography/SRS yielded also a similar sensitivity (82% for each one in terms of the number of detected lesions).
Eight combinations of imaging techniques were evaluated for their sensitivity and cost. The cost of each imaging technique in Euro is shown in Table 4.
| Chest X-ray | 3.29 | |
| Upper abdominal | 8.22 | |
| ultrasonography | ||
| Chest CT-scan | 71.11 | |
| Upper abdominal CT-scan | 71.11 | |
| 48.11 | • for the contrast liquid | |
| Lower abdominal CT-scan | 71.11 | |
| SRS | 1172.42 |
In the four most sensitive combinations, the combination of chest X-ray/upper abdominal ultrasonography/SRS presented the lower cost, 1183.93 Euro vs 1251.75 Euro for chest CT/upper abdominal ultrasonography/SRS, 1294.93 Euro for chest X-ray/upper abdominal CT/SRS and 1362.75 Euro for chest CT/upper abdominal CT/SRS. The related sensitivity rate/cost for the combinations reported above is shown in Figure 6.
Carcinoids were often indolent, asymptomatic, slow growing tumors and clinically silent for years[3]. The vast majority would not cause symptoms until complications (e.g. intestinal obstruction) or symptoms and signs of the carcinoid syndrome occur. This syndrome occured in less than 10% of cases and might be present in patients with midgut carcinoid tumors with liver metastases and also in some patients with foregut carcinoids. Patients with hindgut carcinoids did not exhibit the carcinoid syndrome.
The final diagnosis was not easy, unless bioptic material was examined for the secretory peptide chromogranin or the neuron-specific enolase[19,20].
Due to their multiple localizations and their small size, images of carcinoid tumors were difficult to obtain even when the most sophisticated conventional imaging techniques were applied[21-23]. MRI, CT scan and ultrasonography were very sensitive in the detection of liver metastases, but seemed to be less sensitive in the diagnosis of extrahepatic sites[24-26].
It has been known that carcinoid tumors had a high expression of somatostatin receptors[27,28]. More than 90% of patients with midgut carcinoids expressed somatostatin receptors detected by autoradiography with iodinated somatostatin analogues as ligands, while the somatostatin receptor expression in foregut carcinoid tumors was less frequent[29].
Five different subtypes of somatostatin receptors have been cloned. Somatostatin receptor subtype 2 could bind to somatostatin analogues used in clinical practice with high affinity. Subtypes 3 and 5 had an intermediate affinity while subtypes 1 and 4 had low affinity for the available somatostatin analogues[30].
SRS is a very sensitive method for the demonstration of receptor-positive tumors and their metastases and its diagnostic usefulness in patients with abdominal carcinoid tumors has already been reported[31,32].
In our study SRS imaging visualized the primary or metastatic sites in 22 out of 31 patients with gastrointestinal and pancreatic carcinoid tumors (detection rate 71.0%) and the results were in concordance with other previously published reports[31-33].
Conventional imaging was positive in 19 out of 31 patients (detection rate 61.3%).
Our results demonstrate that SRS, compared with conventional imaging, can provide major additional information.
More interestingly, SRS was positive in 71.4% of the primary tumor sites with a statistically significant difference (P = 0.039) compared with conventional imaging methods. Lebtahi et al[34] reported also similar results (75%) in a similar group of 38 patients. Conventional imaging modalities (ultrasonography and upper abdominal CT) are more sensitive in the detection of hepatic metastases. On the other hand SRS is more sensitive in the detection of extrahepatic metastatic sites and can provide additional information for previous unsuspected localizations. Schillaci et al[35] reported similar results in a group of 18 patients with abdominal carcinoid tumors.
Thus, it is clear that the combination of several conventional imaging techniques with SRS is the method of choice for a better evaluation of patients with carcinoid tumors. For individuals with carcinoid tumors of the digestive tract, gastrointestinal endoscopy is the first line diagnostic tool. Laparotomy can also provide useful information in some cases.
In our study, 2 (chest X-ray/upper abdominal CT scan/SRS and chest CT/upper abdominal CT scan/SRS) out of 8 comb-inations of imaging modalities yielded an overall sensitivity of 88.75% in the detection of primary and metastatic carcinoid sites. The cost was 1294.93 Euro for the combination of chest X-ray/upper abdominal CT scan/SRS and 1362.75 Euro for the combination of chest CT/upper abdominal CT scan/SRS. Thus, there was a benefit of 67.82 Euro using the first one.
In a similar cost-effectiveness analysis of patients with neur-oendocrine tumors, Kwekkeboom et al[36] showed that the comb-ination of SRS, chest radiography and upper abdominal ultrasound led to the detection of carcinoid lesions in all patients in whom carcinoid lesions could be demonstrated by any means, with a sensitivity of 100% in terms of the number of detected lesions, but the cost analysis was made between the proposed imaging strategy and the most sensitive combination of conventional imaging (chest and abdomen CT scans).
| 1. | Wilson JD, Braunwald E, Isselbacher KJ, Petersdorf RG, Mar-tin JB, Fauci S, Root RK (eds). Harrison's principles of internal medicine (12th ed), New york:. McGraw Hill. 1991;. |
| 2. | Pearse AG, Polak JM, Heath CM. Polypeptide hormone production by "carcinoid" apudomas and their relevant cytochemistry. Virchows Arch B Cell Pathol. 1974;16:95-109. [PubMed] |
| 3. | Vinik AI, McLeod MK, Fig LM, Shapiro B, Lloyd RV, Cho K. Clinical features, diagnosis, and localization of carcinoid tumors and their management. Gastroenterol Clin North Am. 1989;18:865-896. [PubMed] |
| 4. | Janson ET, Oberg K. Long-term management of the carcinoid syndrome. Treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol. 1993;32:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Godwin JD. Carcinoid tumors. An analysis of 2,837 cases. Cancer. 1975;36:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502-1522. [PubMed] |
| 7. | Kvols LK, Reubi JC. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Acta Oncol. 1993;32:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Akerström G, Makridis C, Johansson H. Abdominal surgery in patients with midgut carcinoid tumors. Acta Oncol. 1991;30:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, Lameris JS, Reubi JC, Lamberts SW. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 405] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Lamberts SW, Bakker WH, Reubi JC, Krenning EP. Somatostatin-receptor imaging in the localization of endocrine tumors. N Engl J Med. 1990;323:1246-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 317] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Reubi JC, Häcki WH, Lamberts SW. Hormone-producing gastrointestinal tumors contain a high density of somatostatin receptors. J Clin Endocrinol Metab. 1987;65:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Reubi JC, Laissue J, Krenning E, Lamberts SW. Somatostatin receptors in human cancer: incidence, characteristics, functional correlates and clinical implications. J Steroid Biochem Mol Biol. 1992;43:27-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Reubi JC, Kvols L, Krenning E, Lamberts SW. Distribution of somatostatin receptors in normal and tumor tissue. Metabolism. 1990;39:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Bakker WH, Krenning EP, Breeman WA, Kooij PP, Reubi JC, Koper JW, de Jong M, Laméris JS, Visser TJ, Lamberts SW. In vivo use of a radioiodinated somatostatin analogue: dynamics, metabolism, and binding to somatostatin receptor-positive tumors in man. J Nucl Med. 1991;32:1184-1189. [PubMed] |
| 15. | Bakker WH, Krenning EP, Breeman WA, Koper JW, Kooij PP, Reubi JC, Klijn JG, Visser TJ, Docter R, Lamberts SW. Receptor scintigraphy with a radioiodinated somatostatin analogue: radiolabeling, purification, biologic activity, and in vivo application in animals. J Nucl Med. 1990;31:1501-1509. [PubMed] |
| 16. | Joseph K, Stapp J, Reinecke J, Höffken H, Benning R, Neuhaus C, Trautmann ME, Schwerk WB, Arnold R. [Receptor scintigraphy in endocrine gastrointestinal and pancreatic tumors]. Dtsch Med Wochenschr. 1992;117:1025-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 994] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 18. | Kvols LK. Somatostatin-receptor imaging of human malignancies: a new era in the localization, staging, and treatment of tumors. Gastroenterology. 1993;105:1909-1911. [PubMed] |
| 19. | Nash SV, Said JW. Gastroenteropancreatic neuroendocrine tumors. A histochemical and immunohistochemical study of epithelial (keratin proteins, carcinoembryonic antigen) and neuroendocrine (neuron-specific enolase, bombesin and chromogranin) markers in foregut, midgut, and hindgut tumors. Am J Clin Pathol. 1986;86:415-422. [PubMed] |
| 20. | Simpson S, Vinik AI, Marangos PJ, Lloyd RV. Immunohistochemical localization of neuron-specific enolase in gastroenteropancreatic neuroendocrine tumors. Correlation with tissue and serum levels of neuron-specific enolase. Cancer. 1984;54:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Zimmer T, Ziegler K, Bäder M, Fett U, Hamm B, Riecken EO, Wiedenmann B. Localisation of neuroendocrine tumours of the upper gastrointestinal tract. Gut. 1994;35:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Picus D, Glazer HS, Levitt RG, Husband JE. Computed tomography of abdominal carcinoid tumors. AJR Am J Roentgenol. 1984;143:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | McCarthy SM, Stark DD, Moss AA, Goldberg HI. Computed tomography of malignant carcinoid disease. J Comput Assist Tomogr. 1984;8:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Kressel HY. Strategies for magnetic resonance imaging of focal liver disease. Radiol Clin North Am. 1988;26:607-615. [PubMed] |
| 25. | Kisker O, Weinel RJ, Geks J, Zacara F, Joseph K, Rothmund M. Value of somatostatin receptor scintigraphy for preoperative localization of carcinoids. World J Surg. 1996;20:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Shi W, Johnston CF, Buchanan KD, Ferguson WR, Laird JD, Crothers JG, McIlrath EM. Localization of neuroendocrine tumours with [111In] DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM. 1998;91:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | de Herder WW, Lamberts SW. Somatostatin and somatostatin analogues: diagnostic and therapeutic uses. Curr Opin Oncol. 2002;14:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Tomassetti P, Migliori M, Lalli S, Campana D, Tomassetti V, Corinaldesi R. Epidemiology, clinical features and diagnosis of gastroenteropancreatic endocrine tumours. Ann Oncol. 2001;12 Suppl 2:S95-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 29. | Reubi JC, Kvols LK, Waser B, Nagorney DM, Heitz PU, Charboneau JW, Reading CC, Moertel C. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res. 1990;50:5969-5977. [PubMed] |
| 30. | Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427-442. [PubMed] |
| 31. | Kwekkeboom DJ, Krenning EP, Bakker WH, Oei HY, Kooij PP, Lamberts SW. Somatostatin analogue scintigraphy in carci-noid tumors. Eur J Nucl Med. 1993;20:283-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Carnaille B, Nocaudie M, Pattou F, Huglo D, Deveaux M, Marchandise X, Proye C. Scintiscans and carcinoid tumors. Surgery. 1994;116:1118-1121; discussion 1118-1121;. [PubMed] |
| 33. | Scherübl H, Bäder M, Fett U, Hamm B, Schmidt-Gayk H, Koppenhagen K, Dop FJ, Riecken EO, Wiedenmann B. Somatostatin-receptor imaging of neuroendocrine gastroenteropancreatic tumors. Gastroenterology. 1993;105:1705-1709. [PubMed] |
| 34. | Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, Mignon M, le Guludec D. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853-858. [PubMed] |
| 35. | Schillaci O, Scopinaro F, Angeletti S, Tavolaro R, Danieli R, Annibale B, Gualdi G, Delle Fave G. SPECT improves accuracy of somatostatin receptor scintigraphy in abdominal carcinoid tumors. J Nucl Med. 1996;37:1452-1456. [PubMed] |
| 36. | Kwekkeboom DJ, Lamberts SW, Habbema JD, Krenning EP. Cost-effectiveness analysis of somatostatin receptor scintigraphy. J Nucl Med. 1996;37:886-892. [PubMed] |
Edited by Wang XL and Chen WW Proofread by Xu FM