Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3103
Revised: April 6, 2004
Accepted: April 13, 2004
Published online: November 1, 2004
AIM: To study the cell cycle alterations of human hepatoma cell line HepG2 in vitro after 60Co γ-irradiation and further to examine the mechanisms underlying the enhancement of radiosensitivity to γ-irradiation in HepG2 transiently transfected with wild type p27kip1.
METHODS: The proliferation of HepG2 cells was evaluated with MTT assay, and the cell cycle profile and apoptosis were assessed by cell morphology, DNA fragmentation analysis and flow cytometry. HepG2 cells were transfected with p27kip1 wild type by using Lipofectamine (LF2000), and the expression and subcellular localization of p27kip1 in HepG2 were detected by immunocytochemistry.
RESULTS: 60Co γ-irradiation inhibited the growth of HepG2 cells in a dose-dependent manner. Apoptosis of HepG2 cells was induced 48 h after γ ray exposure. Furthermore research was carried out to induce exogenous expression of p27kip1 in HepG2. The expression of p27kip1 induced G0/G1 phase arrest in HepG2 cells. The overexpression of p27kip1 enhanced 60Co γ-irradiation-induced radiosensitivity in HepG2 cells.
CONCLUSION: Overexpression of p27kip1 is a rational approach to improve conventional radiotherapy outcomes, which may be a possible strategy for human hepatoma therapy.
- Citation: Guan XX, Chen LB, Ding GX, De W, Zhang AH. Transfection of p27kip1 enhances radiosensitivity induced by 60Co γ-irradiation in hepatocellular carcinoma HepG2 cell line. World J Gastroenterol 2004; 10(21): 3103-3106
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3103.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3103
Hepatocellular carcinoma (HCC) is a relatively common malignancy, ranking fifth in frequency on a worldwide basis and causing more than one million deaths annually[1,2] and there has been a progressive increase in the number of hepatoma cases over the past two decades[3]. Unfortunately, most of the cases of hepatoma are not curable because extensive resection is not possible. Though many approaches, such as transarterial chemoembolization (TACE), percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), radiotherapy and liver transplantation have been developed to treat it, and the effective and survival rates are increased, a large number of patients would die from recurrence and metastasis[4-8]. It is well known that improving the overall therapeutic effects of liver cancer depends on the combined therapies. The purpose of combined interventional therapies for HCC is to increase their therapeutic efficiencies and to reduce the side effects and complications.
Radiotherapy presents another interesting option for the treatment of HCC amidst the wide array of non-surgical modalities available[11] and experimental and clinical studies have been reported that gene therapy is one of the more promising approaches for patients with advanced liver tumours[9-12].
It is unknown about the combination of radiotherapy and gene therapy, especially the relationship between p27kip1 and radiosensitivity, although p27kip1 is a target for cancer therapeutics[13]. p27kip1 is a key molecule in cell cycle control because of their specific and periodic expression during cell cycle progression. Knowledge of the function of cell cycle checkpoints in tumour cells may be important to develop treatment strategies for human cancers. Recent studies indicate that mutations in the p27kip1 gene have not been seen in many tumors including human hepatoma[14]. Down-regulation and mislocalization expression of p27kip1 have recently been found to be associated with a poor prognosis in patients with hepatoma[15]. However, the role of p27kip1 expression and gamma irradiation-induced apoptosis in human hepatoma cells has not been examined previously.
The purpose of this study was to investigate the function of p27kip1 and 60Co γ-irradiation in the HepG2 cell cycle progress and apoptosis, and then to examine the molecular mechanisms of radiosensitivity induced by p27kip1 and gamma irradiation in human hepatoma cells, focusing on the possibility that it might act, at least in part, by increasing the expression of p27kip1 in human hepatoma cells. This study may help us to understand radiosensitivity and develop a new treatment strategy.
RPMI-1640 medium and Lipofectamine (LF2000) were purchased from GIBCO. Anti-Flag (M2) was purchased from Sigma. FITC conjugated-IgG was purchased from Santa Cruz Biotechnology. The plasmid containing p27kip1 was kindly provided by Dr. Keiichi Nakayama at Kyushu University, Japan.
Human hepatoma cell line, HepG2, was routinely maintained in RPMI-1640 medium supplemented with 100 mL/L heat-inactivated fetal bovine serum at the 100 mL/L concentration and incubated in 100 U/mL penicillin-streptomycin in 50 mL/L CO2 in air at 37 °C with 50 mL/L CO2. Hepatoma cells were plated into 6-well culture plates (5 × 10 4 cells/well) and exposed to 0, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 Gy of γ-irradiation from a telecobalt therapy source at a dose rate of 5.0 cGy/minute.
Transfection of the cells with wild type p27kip1 cDNA constructs was performed using Lipofectamine (LF2000) reagent according to the manufacturer’s instructions. Briefly, cells were plated in a 6-well plate at a density of 5 × 10 4/well and incubated overnight in RPMI-1640 supplemented with 100 mL/L FCS. The cDNA constructs encoding p27kip1 were diluted in RPMI-1640 (100 µL) and then mixed with the transfection solution for 15 min. After washed twice with phosphate-buffered saline (PBS) to remove serum, the cells were incubated with the transfection mixture at 37 °C for 4 h and then refreshed with RPMI-1640 medium.
Cell proliferation was measured by MTT assay. After HepG2 cells were treated with different dose gamma-irradation for indicated time and dose, then 10 µL MTT (5 mg/mL) was added to each well and incubated for an additional 4 h, and then the liquid in the wells was evaporated. To dissolve the formazam, 200 µL of DMSO was added. Control wells were treated with 1 g/L DMSO alone. The absorbance was detected in the microplate reader 550 model at 570 nm wavelength. Growth inhibition was equal to (1-absorbance of the treated wells)/(absorbance of the control wells) × 100%.
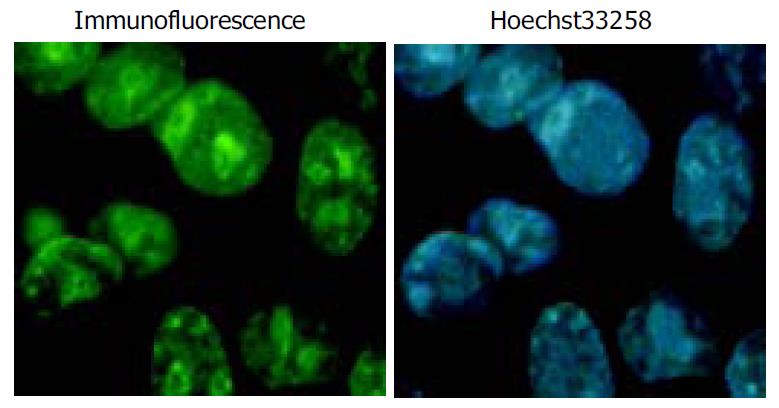
Immunohistochemical staining was performed to determine the expression of Flag-P27kip1 fusion protein. HepG2 cells transiently expressing p27kip1 were grown on a glass coverslip in a 6-well plate for 24 h after transfetion. The cells on the coverslips were fixed in 20 g/L paraformaldehye at room temperature for 5 min on the next day of transfection, and sequentially incubated for 60 min with anti-mouse Flag (M2) and then FITC-conjugated second antibody. The slides were lightly counterstained with Hoechst 33258, washed with water and then mounted. Finally coverslips were mounted with other glass slides, the cells were examined by immunofluorescence microscopy at the excitation/emission wavelengths of 488 nm and 520 nm alternatively.
HepG2 cells were plated onto a 6-well plate (5 × 10 4 cells/well) in RPMI-1640 containing 100 mL/L FBS and grown overnight to allow cell attachment. They were then treated with irradiation, harvested, fixed with 700 mL/L ethanol, centrifuged, resuspended in 400 µL of PBS, and 2 mg/mL RNase was added to avoid double-stranded RNA staining, then stained with 400 µL of 0.1 mg/mL propidium iodide (PI). The cell suspension was filtered through a 60-µm Specrta/Mesh nylon filter. Samples of 20 000 cells were then analyzed for DNA histograms and cell cycle phase distributions by flow cytometer using a FACSCalibur instrument (Becton Dickinson), and the data were analyzed by a CELLQuest computer program.
The integrity of DNA was assessed by agarose gel electrophoresis. Cells (1 × 10 6) were centrifuged for 5 min at 3 000 r/min, washed once with PBS, and cell pellets were resuspended in 100 µL of lysis buffer (50 mmol/L Tris-HCl, pH8.0, 10 mmol/L tetraacetic acid, 4 g/L SDS, 0.5 g/L proteinase K) and incubated for 8 h at 50 °C, then 10 µL of 0.5 g/L RNase A was added. The samples were incubated for 1 h at 50 °C and heated to 70 °C for 5 min, then 100 µL of phenol: Chloroform:Isopropanol (25:24:1) was added. After centrifugation, the supernatants were transferred to new tubes, and twice volume ethanol (ice cold) was added. After centrifugation, the pellets were solubilized in TE buffer and loaded on 18 g/L agarose gel for electrophoresis. The gel was stained with ethidium bromide, and photographed with UV illumination.
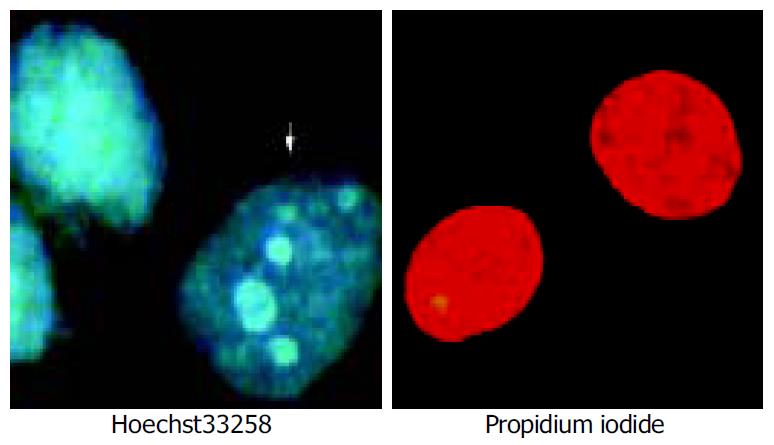
Apoptosis and death cells were identified by Hoechst 33258-PI counterstaining[16]. Briefly, cell pellets (1 × 10 9) were suspended by 100 µL PBS containing Hoechst 33258 at the concentration of 1 µL/mL. The cells were incubated at 37 °C for 7 min, and then centrifuged. The cell pellets were resuspended in 100 µL staining solution containing PI at the concentration of 5 µg/mL. The stained cells were analyzed using a fluorescence microscope. Samples of 200 cells were then analysed.
Data were represented as mean ± SD. Differences were evaluated by SPSS10.0 software. P≤ 0.05 was considered statistically significant.
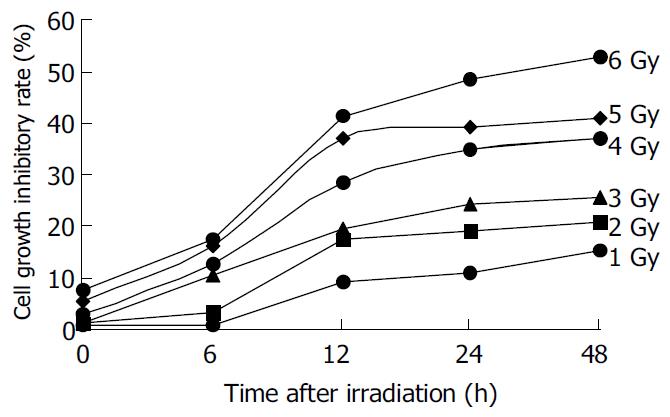
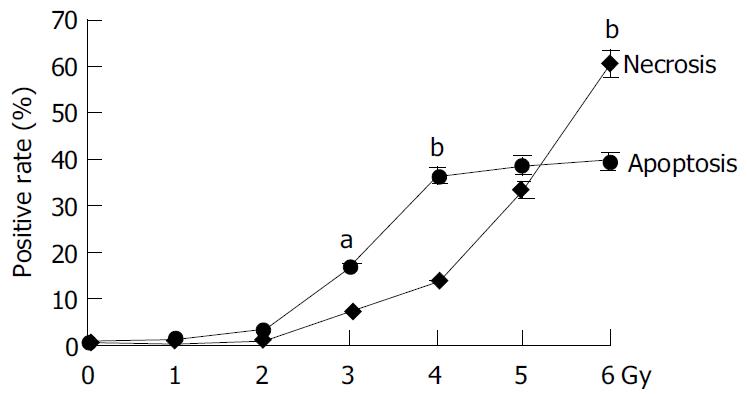
The irradiation of HepG2 cells caused a dose-dependent cell growth inhibition, and a maximum inhibitory effect was observed at 6 Gy. MTT assay showed that irradiation had anti-proliferative effects on HepG2 cells in a dose-dependant manner. At the dose of 0, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 Gy, the inhibitory rate was 2.62% ± 0.18%, 26.85% ± 0.21%, 54.11% ± 0.47%, 57.19% ± 0.54%, 60.26% ± 0. 53% and 67.12% ± 0.65% respectively (Figure 1). The cell growth inhibitory effect was due to apoptosis or necrosis induced by excessively high dose irradiation. The counterstaining of PI and Hoechst 33258 proved to be an excellent probe to distinguish apoptotic cells from necrotic cells. Live and apoptotic cells were only probed by Hoechst 33258 (blue in color) and exclusion of propidium iodide due to plasma membrane integrity. The morphological features of apoptotic cells were cell shrinkage, nuclear condensation and genomic fragmentation down to the size of individual nucleosome units. On the contrary, necrotic cells were probed by PI (red in colors) (Figure 2). The apoptotic rate reached the top at the dose of 4.0 Gy, when irradiated by 6.0 Gy, most of HepG2 cells were in necrotic state (Figure 3).
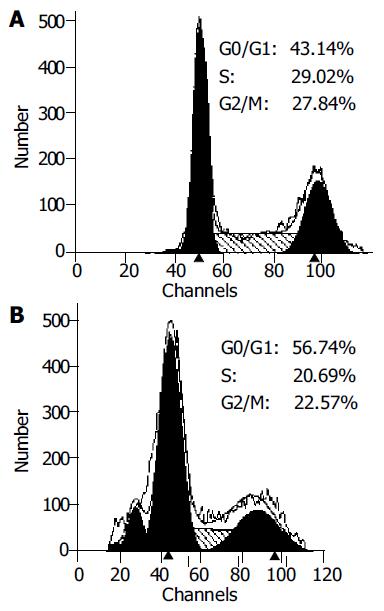
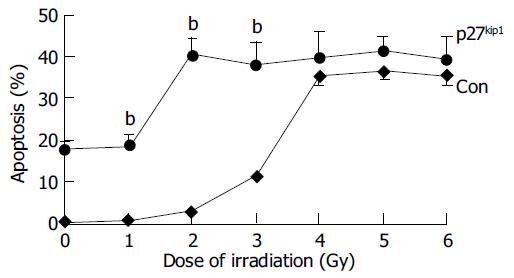
Overexpression of p27kip1 protein was observed in transfected cells (Figure 4). As a result, the proliferation of HepG2 cells was greatly inhibited and cell cycle was arrested in G1 phase after exogenous p27kip1 expression (Figure 5). Further results showed that overexpression of p27kip1 enhanced radiosensitivity in HepG2 cells induced by 60Co γ-irradiation, and 2.0 Gy irradiation induced maximum apoptotic rate by 40.6% in HepG2 cells transfected with p27Kip1, whereas HepG2 cells transfected empty vector, only 4.0 Gy reached the maximum apoptotic rate of 35.3% (Figure 6). Apoptosis of HepG2 cells indicated by flow cytometry and Hoechest33258 staining was then confirmed by DNA fragmentation, which was visualized as the characteristic oligonucleosome-sized fragmentation in ethidium bromide after DNA agarose gel electrophoresis (date not shown).
HCC remains one of the most difficult tumors to treat. Primary HCC is the second most common cancer and the leading cause of cancer deaths behind gastric cancer in China. Surgical resection has been accepted as the only curative therapy for primary liver cancer. Unfortunately, most patients are surgically unresectable, and are sometimes recommended to receive non-surgical therapies, including radiotherapy, radiofrequency hyperthermia, genetherapy or combination of the above methods. Radiation therapy has been commonly used in the treatment of unamenable human hepatoma. Unfortunately, the cause of this radiosensitization has not met expectations fully. New approches that may reduce side-effects and provide good quality of life are required. Thus, it is imperative to develop new and effective treatments, such as gene therapy, in order to treat this disease.
Gene therapy is one of the more promising approaches for patients with advanced liver tumour. Experimental and clinical studies have reported that gene therapy and molecular prevention are becoming a part of patient management and would eventually complement or in part replace the existing therapeutic and preventive strategies[17]. Recent researches have indicated that p27kip is a new target for gene therapy and p27kip1 is a new suitable candidate for gene therapy[9,18]. The protein p27kip1 is an important factor that regulates cell cycle progression and apoptosis. Mutations in p27kip1 gene have not been seen in many tumors including human hepatoma. Down-regulation and mislocalized expression have recently been found to be associated with poor prognosis in patients with hepatoma. Therefore, regulation of p27kip1 activity is a new strategy for hepatoma therapy.
Pretreatment of hepatocellular carcinoma cells with overexpression of p27kip1 protein before irradiation enhanced the cell-killing effect of irradiation. Interaction with moderate doses of radiation caused a substantial increase in tumor cell killing. The beneficial effect of this interaction was further evidenced by the significant increase in the number of apoptic cells. The HepG2 cells treated with p27kip1 and exposed to 6.0 Gy showed a maximum apoptosis percentage when compared to the other irradiation doses or p27kip1 transfection. Our results showed that overexpression of p27kip1 could enhance the radiosensitivity in HepG2 cells. Overexpression of p27kip1 in HepG2 cells cound sensitize cells to ionizing radiation. Recent advances have been made in the understanding of molecular events following cell exposure to ionizing radiation. Our results suggest that p27kip1 protein could be used to modulate radio-induced cellular responses.
However, the molecular machanism of p27kip1 in radiosensitivity induced by 60Co γ-irradiation is still unclear, and further studies are needed to identify the molecular mechanisms for the radiosensitizing activity, with emphasis on the study of p27kip1 in cell cycle progress and radiosensitivity. Our results here provide some experimental evidences that overexpression of p27kip1 increases the radiosensitivity of gamma irradiation. It may help us understand radiosensitivity and develop strategies for liver cancer.
| 1. | Hao XS, Chen KX, Wang PP, Rohan T. Changes in survival patterns in urban Chinese patients with liver cancer. World J Gastroenterol. 2003;9:1212-1215. [PubMed] |
| 2. | Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 672] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87-107, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 5. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Huang YH, Wu JC, Lui WY, Chau GY, Tsay SH, Chiang JH, King KL, Huo TI, Chang FY, Lee SD. Prospective case-controlled trial of adjuvant chemotherapy after resection of hepatocellular carcinoma. World J Surg. 2000;24:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 681] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 8. | Keng GH, Sundram FX. Radionuclide therapy of hepatocellular carcinoma. Ann Acad Med Singapore. 2003;32:518-524. [PubMed] |
| 9. | Schmitz V, Wang L, Barajas M, Gomar C, Prieto J, Qian C. Treatment of colorectal and hepatocellular carcinomas by adenoviral mediated gene transfer of endostatin and angiostatin-like molecule in mice. Gut. 2004;53:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Shiba H, Okamoto T, Futagawa Y, Ohashi T, Eto Y. Efficient and cancer-selective gene transfer to hepatocellular carcinoma in a rat using adenovirus vector with iodized oil esters. Cancer Gene Ther. 2001;8:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Iwazawa T, Chau GY, Mori T, Dookeran KA, Rubin JT, Watkins S, Robbins PD, Lotze MT, Tahara H. Potent antitumor effects of intra-arterial injection of fibroblasts genetically engineered to express IL-12 in liver metastasis model of rat: no additional benefit of using retroviral producer cell. Cancer Gene Ther. 2001;8:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Humphreys MJ, Ghaneh P, Greenhalf W, Campbell F, Clayton TM, Everett P, Huber BE, Richards CA, Ford MJ, Neoptolemos JP. Hepatic intra-arterial delivery of a retroviral vector expressing the cytosine deaminase gene, controlled by the CEA promoter and intraperitoneal treatment with 5-fluorocytosine suppresses growth of colorectal liver metastases. Gene Ther. 2001;8:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Sasaki T, Katayose Y, Suzuki M, Yamamoto K, Shiraso S, Mizuma M, Unno M, Takeuchi H, Lee CT, Matsuno S. Adenovirus expressing mutant p27kip1 enhanced apoptosis against cholangiocarcinoma than adenovirus-p27kip1 wild type. Hepatogastroenterology. 2004;51:68-75. [PubMed] |
| 14. | Ponce-Castañeda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J. p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 1995;55:1211-1214. [PubMed] |
| 15. | Fiorentino M, Altimari A, Ravaioli M, Gruppioni E, Gabusi E, Corti B, Vivarelli M, Bringuier PP, Scoazec JY, Grigioni WF. Predictive value of biological markers for hepatocellular carcinoma patients treated with orthotopic liver transplantation. Clin Cancer Res. 2004;10:1789-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1368] [Cited by in RCA: 1415] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 17. | Blum HE. Molecular therapy and prevention of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2003;2:11-22. [PubMed] |
| 18. | Katner AL, Gootam P, Hoang QB, Gnarra JR, Rayford W. A recombinant adenovirus expressing p7(Kip1) induces cell cycle arrest and apoptosis in human 786-0 renal carcinoma cells. J Urol. 2002;168:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Co-correspondents: Ai-Hua Zhang
Edited by Zhang JZ and Wang XL Proofread by Xu FM