Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3099
Revised: March 29, 2004
Accepted: April 6, 2004
Published online: November 1, 2004
AIM: To determine the role of interferon (IFN) with or without ribavirin in preventing or delaying hepatocellular carcinoma (HCC) development in patients with hepatitis C virus (HCV) related cirrhosis. Data on the preventive effect of IFN plus ribavirin treatment are lacking.
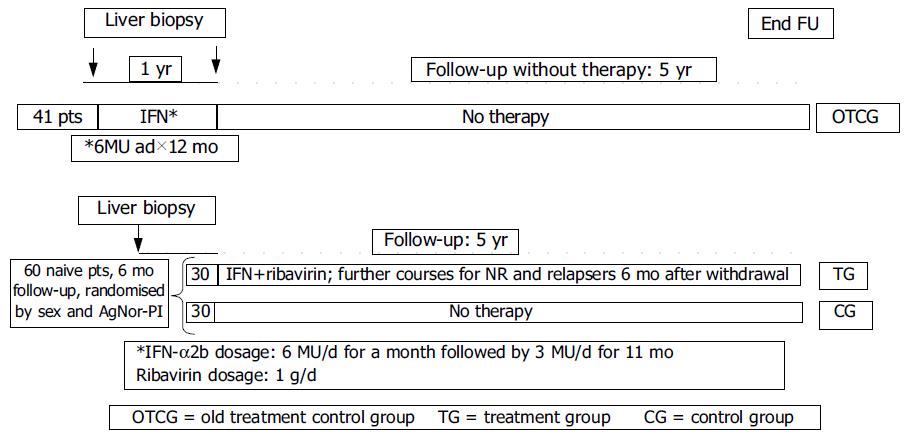
METHODS: A total of 101 patients (62 males and 39 females, mean age 55.1 ± 1.4 years) with histologically proven HCV related liver cirrhosis plus compatible biochemistry and ultrasonography were enrolled in the study. Biochemistry and ultrasonography were performed every 6 mo. Ultrasound guided liver biopsy was performed on all detected focal lesions. Follow-up lasted for 5 years. Cellular proliferation, evaluated by measuring Ag-NOR proteins in hepatocytes nuclei, was expressed as AgNOR-Proliferative index (AgNOR-PI) (cut-off = 2.5). Forty-one patients (27 males, 14 females) were only followed up after the end of an yearly treatment with IFN-alpha2b (old treatment control group = OTCG). Sixty naive patients were stratified according to sex and AgNOR-PI and then randomized in two groups: 30 were treated with IFN-alpha2b + ribavirin (treatment group = TG), the remaining were not treated (control group = CG). Nonresponders (NR) or relapsers in the TG received further IFN/ribavirin treatments after a 6 mo of withdrawal.
RESULTS: AgNOR-PI was significantly lowered by IFN (P < 0.001). HCC incidence was higher in patients with AgNOR-PI > 2.5 (26% vs 3%, P < 0.01). Two NR in the OTCG, none in the TG and 9 patients in the CG developed HCC during follow-up. The Kaplan-Mayer survival curves showed statistically significant differences both between OTCG and CG (P < 0.004) and between TG and CG (P < 0.003).
CONCLUSION: IFN/ribavirin treatment associated with re-treatment courses of NR seems to produce the best results in terms of HCC prevention. AgNOR-PI is a useful marker of possible HCC development.
- Citation: Francesco A, Esterita A, Giovanni N, Davide T, Silvia G, Anna M, Francesca L, Marco M, Mariarosa T, Antonio C, Constance M, Davide F, Enrico R, Massimo D, Giuseppe M. Interferon plus ribavirin and interferon alone in preventing hepatocellular carcinoma: A prospective study on patients with HCV related cirrhosis. World J Gastroenterol 2004; 10(21): 3099-3102
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3099
A number of studies have reported that treatment of HCV related cirrhosis might have a preventive effect on hepatocellular carcinoma development[1-15]. This has been recently confirmed by a meta-analysis concluding that “Interferon (IFN) prevents or delays the development of hepatocellular carcinoma (HCC) in patients with HCV-related cirrhosis, the magnitude of the overall effect is low and the benefit may be partly due to spurious associations. The preventive effect seems more evident among sustained responders to IFN[16]. However, with old interferon schedules, sustained responders did not exceed 10%-15% of treated patients[1-15].
The response rate to IFN has changed since the introduction of ribavirin[17], the induction protocols[18] and, finally, the pegylated interferons[19]. Data on the preventive effect on HCC of more powerful therapeutic schemes are lacking.
Our study was started in 1997 with the purpose of assessing the efficacy of interferon (given with an induction protocol) plus ribavirin (the gold standard treatment at that time) in the prevention of HCC development.
A total of one hundred and one consecutive patients (62 males and 39 females, mean age 55.1 ± 1.4 years) with HCV related liver cirrhosis diagnosed by liver biopsy plus compatible biochemical parameters and ultrasonographic signs of portal hypertension were enrolled in the study. The baseline histologic activity of all patients was moderate to severe.
Forty one subjects (27 males and 14 females, mean age 55.0 ± 1.1 years), who were ending a 12-mo IFN course were followed-up without any other treatment (old treatment control group = OTCG). All patients of OTCG underwent liver biopsy at the end of treatment, while still on IFN.
The other 60 untreated patients (35 males and 25 females, mean age 55.7 ± 1.7 years) were randomized in two groups of 30 subjects stratified according to sex and silver stained nucleolar organizer region - proliferative index (AgNOR-PI). Thirty were treated with IFN + ribavirin (treatment group = TG) and the remaining received no drugs (control group = CG). Nonresponders or relapsers to IFN/ribavirin received further courses of treatment after a 6 mo withdrawal. All patients were followed up for five years.
The study was carried out according to the Helsinki protocol and all patients gave their written informed consent.
Ultrasonographies, blood cell count, α1-fetoprotein, γ GT, transaminases, PT, total protein and their fractions were performed every 3 mo in all patients. Additional tests were performed in patients under active treatment: blood cell count every 10 d for two months and then monthly; transaminases, urea, creatinine and uric acid were tested monthly. When focal lesions were detected by ultrasound (US), US-guided liver biopsy was performed.
The 41 patients in OTCG were treated with IFNα-2b 6 MU/d for a month followed by 3 MU/d for 11 mo. The 30 patients in TG received the following α-2b schedule: 6 MU/d for a mo then 3 MU/d for 11 mo plus ribavirin 1 g/d for 12 mo. IFN and ribavirin dose reductions were made according to the biochemistry and tolerance of each patient. However, a total dose equal to or greater than 540 MU and 400 mg of ribavirin per day were considered suitable. Nonresponders and/or relapsers received further IFN treatment courses after a 6-mo withdrawal.
Ultrasound guided liver biopsies were fixed in 40 g/L formaldehyde solution for 6 h and embedded in paraffin wax. Four 4 μm thick sections were cut from routinely processed paraffin blocks. Hematoxylin-eosin, silver impregnation, Pearl’s staining were performed to define the severity of parenchymal, portal and periportal inflammation and the stage of disease by evaluating fibrosis and the presence of stainable iron into the liver. Histology was evaluated by two blinded independent observers according to Scheuer score.
The AgNOR staining was performed on routine sections of liver tissue on poly-lysine pretreated slides after immersion in xylene and ethanol. After progressive re-hydration sections were covered with plastic resistant to high temperature, put in a sodium citrate (100 g/L, pH6.0) solution and boiled in pressured ovens (120 °C for 20 min). Then, sections were stained by silver impregnation in a gelatine solution (formic acid 10 mL/L and silver nitrate 500 g/L, 100:2 v/v) according to Ploton[20], for 10 min, at 37 °C. Quantitative analysis of Ag-NOR proteins was made by measuring silver-stained areas (μm2) within nuclei present in 50 consecutive microscopic fields (40 × magnification) using a specific computer-assisted imaging software on biopsy specimen sections (VIDAS, Kontron Elecktronic, Germany). The percentage of hepatocytes with an AgNOR area > 7 μm2 (indicative of a proliferative state) was expressed as proliferative index (AgNOR-PI) (cut-off = 2.5%).
Results were expressed as mean ± SE. The statistical analysis was carried out according to the intention to treat analysis. Wilcoxon test was used when appropriate and the Kaplan-Mayer model was applied to the evaluation of survival probability.
Demographic and biochemical characteristics of the patients at enrollment are shown in Table 1. The three groups were comparable for age, sex, biochemical parameters, genotype distribution and AgNOR-PI.
| OTCG | TG | CG | P < | |
| M:F ratio | 27:14 | 17:13 | 18:12 | NS |
| Age (yr) | 55.3 ± 1.8 | 54.6 ± 2.1 | 57.2 ± 2.0 | NS |
| AST (U/L) | 67.1 ± 6.6 | 61.9 ± 7.2 | 79.4 ± 8.4 | NS |
| ALT (U/L) | 92.5 ± 10.8 | 79.8 ± 8.7 | 91.8 ± 9.1 | NS |
| γ GT (U/L) | 56.5 ± 7.2 | 52.1 ± 7.3 | 65.2 ± 9.4 | NS |
| Albumin (g/dL) | 4.2 ± 0.07 | 4.2 ± 0.07 | 4.1 ± 0.06 | NS |
| α 1feto (ng/mL) | 6.8 ± 1.25 | 8.4 ± 2.36 | 6.3 ± 1.0 | NS |
| HCV1b | 63% | 67% | 65% | NS |
| AgNOR-PI (%) | 20.1 ± 2.35 | 19.6 ± 2.84 | 18.2 ± 2.6 | NS |
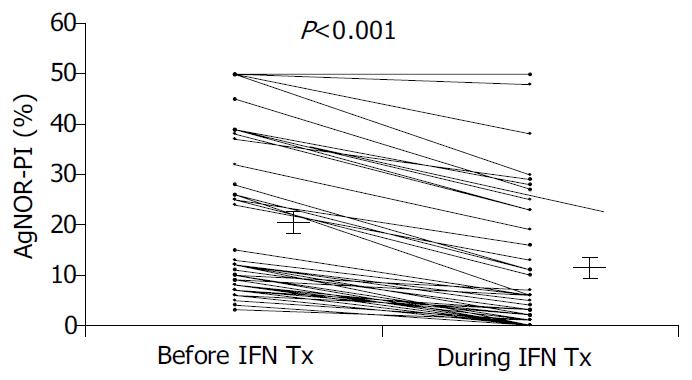
A significant reduction in AgNOR-PI was observed after IFN-treatment (Figure 2).
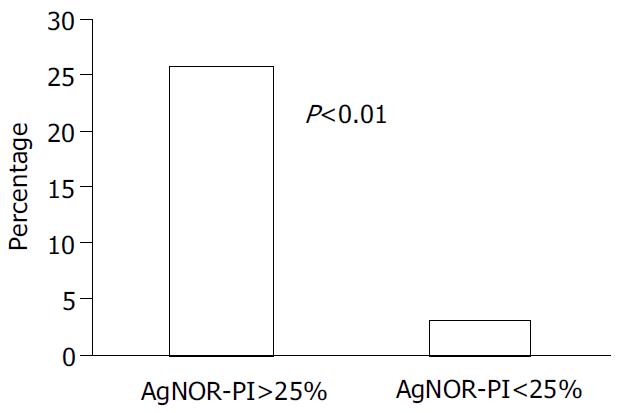
A significant difference in HCC development was observed according to AgNOR-PI: 9 out of 35 (26%) with basal AgNOR-PI > 2.5% vs 2 out of 66 (3%) with basal AgNOR-PI < 2.5% (P < 0.01) (Figure 3).
Six months after IFN withdrawal 8 out of 19 responders (24.5%) achieved sustained response (6 with genotype 2 or 3 and 2 with genotype 1) in the OTCG. Twenty one out of 30 patients in TG (70%) achieved a virological response that was sustained in 13 (43%) (9 genotype 2 and 4 genotype 1). None of the re-treated patients showed a sustained response.
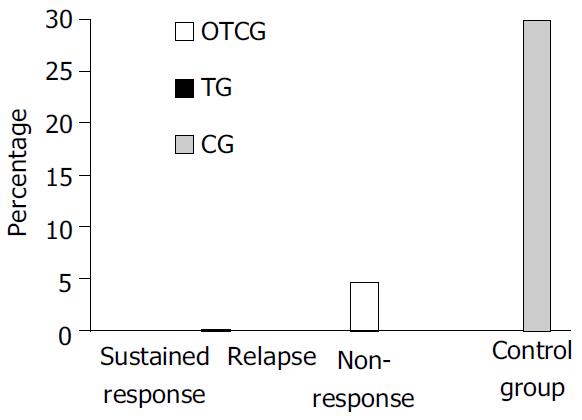
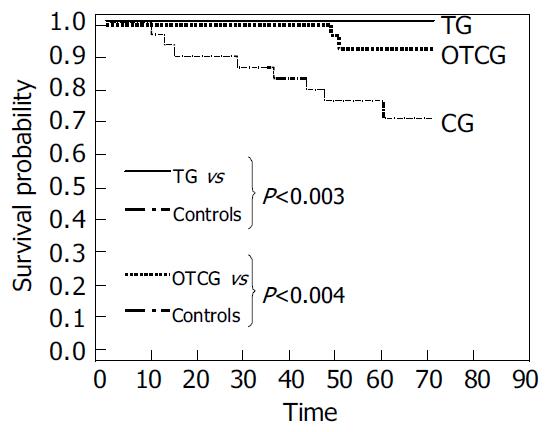
Two nonresponders in OTCG developed HCC during 5 years of follow-up after about 50 mo from interferon withdrawal. No subject in TG while 9 (30%) patients in CG developed HCC (Figure 4). The Kaplan-Mayer survival model showed statistically significant differences both between OTCG and CG (P < 0.004) and between TG and CG (P < 0.003) (Figure 5). The HCC annual rate of incidence in the CG was 5%.
The present data add new evidence on the clinical efficacy of IFN re-treatment of cirrhotic patients and show the usefulness of AgNOR-PI. The ability of IFN to prevent HCC development is evident both alone and in combination with ribavirin.
Previous observations in patients with chronic hepatitis C[21-24], with or without cirrhosis, reported that re-treatment with IFN was more effective than single courses in preventing HCC appearance. However, in these studies[21-24], the vast majority of patients had chronic hepatitis and no conclusions could be drawn on cirrhotic patients. We extended those observations to patients with HCV related cirrhosis. In accordance with a previous study[1], a single course of IFN did not seem to be protective toward HCC appearance in a long term follow-up. In fact, in the two nonresponder patients of OTCG, HCC developed after about 50 mo, suggesting that the protective effect of IFN may vanish over time. This hypothesis is strengthened by the observation that re-treatment of nonresponders in the TG prevented HCC development during the 5 years of follow-up.
It is interesting to note that no statistically significant difference was observed in survival between TG and OTCG. This may suggest that the addition of ribavirin to IFN did not add any significant benefit in cirrhotics. However, the number of patients was probably not sufficient to appreciate any possible difference coming from the higher rate of sustained response obtained with the combination treatment. However, it remains that the key to HCC prevention is treatment with interferon that might be helpful even after a curative resection of HCC[25].
Our study also showed that AgNOR-PI was a useful marker of hepatocyte regeneration which is able to predict a possible evolution to HCC. Furthermore, the two patients who developed HCC in the OTCG were those with the highest AgNOR-PI without improvement after treatment. This underlines the relevance of the index in the clinical setting, particularly in nonresponders to IFN. In fact, it may restrict the need of a strict surveillance only to those patients with a higher risk of developing HCC.
Previous observations with different techniques have shown that high hepatocyte proliferation is associated with HCC development. A recent paper evaluating nucleolar hypertrophy in patients with HBV and HCV related cirrhosis reported the index was significantly predictive of HCC development only in patients with HBV related cirrhosis[30]. In patients with HCV related cirrhosis the index was not significantly related with HCC development, although a trend could be appreciated. A much larger and more homogeneous population of patients with HCV related cirrhosis (only Child A) in our study could account for the different results between the two studies.
In conclusion, the preventive effect of IFN on HCC development in HCV related cirrhosis is confirmed. Furthermore, a more efficacious treatment associated with re-treatment courses of nonresponders seems to produce the best results in term of HCC prevention. AgNOR-PI is a useful marker of hepatocyte proliferation that identifies patients at higher risk of developing HCC.
| 1. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 607] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Mazzella G, Accogli E, Sottili S, Festi D, Orsini M, Salzetta A, Novelli V, Cipolla A, Fabbri C, Pezzoli A. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV-related liver cirrhosis. J Hepatol. 1996;24:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 209] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Bruno S, Silini E, Crosignani A, Borzio F, Leandro G, Bono F, Asti M, Rossi S, Larghi A, Cerino A. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology. 1997;25:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 247] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 960] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 5. | Effect of interferon-alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. International Interferon-alpha Hepatocellular Carcinoma Study Group. Lancet. 1998;351:1535-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 169] [Reference Citation Analysis (0)] |
| 6. | Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, Maeda Y, Shirai Y, Fukuzaki T, Kaji I. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Gramenzi A, Andreone P, Fiorino S, Cammà C, Giunta M, Magalotti D, Cursaro C, Calabrese C, Arienti V, Rossi C. Impact of interferon therapy on the natural history of hepatitis C virus related cirrhosis. Gut. 2001;48:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Serfaty L, Aumaître H, Chazouillères O, Bonnand AM, Rosmorduc O, Poupon RE, Poupon R. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 278] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 9. | Sofia S, Casali A, Buscarini E, Castagnetti E, Rapaccini GL, Levantesi L, Salmi A, Boccia S, Miglio F, Ricca Rossellini S. Effect of lymphoblastoid IFN in the treatment of liver cirrhosis and prevention of HCC. Ital J Gastroenterol Hepatol. 1998;30:A67. |
| 10. | Benvegnù L, Chemello L, Noventa F, Fattovich G, Pontisso P, Alberti A. Retrospective analysis of the effect of interferon therapy on the clinical outcome of patients with viral cirrhosis. Cancer. 1998;83:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Shioda A, Moriyama M, Kaneko M, Shimizu T, Gotou I, Tanaka N, Ookubo H, Arakawa Y. Long term prognosis of hepatocel-lular carcinoma developing after treatment of interferon in pa-tients with chronic hepatitis C and liver cirrhosis. Hepatology. 1999;30:A268. |
| 12. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 781] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 13. | Mura D, Delliperi R, Fastame L, Carlini A, Cussu PA, Pisanu G, Dore MP, Realdi G. Five years follow-up after interferon therapy in HCV positive compensated cirrhosis. Ital J Gastroenterol Hepatol. 1998;30:A114. |
| 14. | Valla DC, Chevallier M, Marcellin P, Payen JL, Trepo C, Fonck M, Bourliere M, Boucher E, Miguet JP, Parlier D. Treatment of hepatitis C virus-related cirrhosis: a randomized, controlled trial of interferon alfa-2b versus no treatment. Hepatology. 1999;29:1870-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 15. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Cammà C, Giunta M, Andreone P, Craxì A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001;34:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 17. | Davis GL. Combination treatment with interferon and ribavirin for chronic hepatitis C. Clin Liver Dis. 1999;3:811-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Vrolijk JM, Bekkering FC, Brouwer JT, Hansen BE, Schalm SW. High sustained virological response in chronic hepatitis C by combining induction and prolonged maintenance therapy. J Viral Hepat. 2003;10:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | McHutchison JG, Fried MW. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin Liver Dis. 2003;7:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Ploton D, Menager M, Jeannesson P, Himber G, Pigeon F, Adnet JJ. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986;18:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 699] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Hino K, Kitase A, Satoh Y, Fujiwara D, Yamaguchi Y, Korenaga M, Shingai Y, Konishi T, Yamashita S, Uchida K. Interferon retreatment reduces or delays the incidence of hepatocellular carcinoma in patients with chronic hepatitis C. J Viral Hepat. 2002;9:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tanaka H, Tsukuma H, Kasahara A, Hayashi N, Yoshihara H, Masuzawa M, Kanda T, Kashiwagi T, Inoue A, Kato M. Effect of interferon therapy on the incidence of hepatocellular carcinoma and mortality of patients with chronic hepatitis C: a retrospective cohort study of 738 patients. Int J Cancer. 2000;87:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Sone Y, Hisanaga Y. The effect of retreatment with interferon-alpha on the incidence of hepatocellular carcinoma in patients with chronic hepatitis C. Cancer. 2000;88:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Takimoto M, Ohkoshi S, Ichida T, Takeda Y, Nomoto M, Asakura H, Naito A, Mori S, Hata K, Igarashi K. Interferon inhibits progression of liver fibrosis and reduces the risk of hepatocarcinogenesis in patients with chronic hepatitis C: a retrospective multicenter analysis of 652 patients. Dig Dis Sci. 2002;47:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Sun HC, Tang ZY. Preventive treatments for recurrence after curative resection of hepatocellular carcinoma--a literature review of randomized control trials. World J Gastroenterol. 2003;9:635-640. [PubMed] |
| 26. | Tarao K, Ohkawa S, Shimizu A, Harada M, Nakamura Y, Ito Y, Tamai S, Hoshino H, Inoue T, Kanisawa M. Significance of hepatocellular proliferation in the development of hepatocellular carcinoma from anti-hepatitis C virus-positive cirrhotic patients. Cancer. 1994;73:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Ballardini G, Groff P, Zoli M, Bianchi G, Giostra F, Francesconi R, Lenzi M, Zauli D, Cassani F, Bianchi F. Increased risk of hepatocellular carcinoma development in patients with cirrhosis and with high hepatocellular proliferation. J Hepatol. 1994;20:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Sangiovanni A, Colombo E, Radaelli F, Bortoli A, Bovo G, Casiraghi MA, Ceriani R, Roffi L, Redaelli A, Rossini A. Hepatocyte proliferation and risk of hepatocellular carcinoma in cirrhotic patients. Am J Gastroenterol. 2001;96:1575-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Donato MF, Arosio E, Del Ninno E, Ronchi G, Lampertico P, Morabito A, Balestrieri MR, Colombo M. High rates of hepatocellular carcinoma in cirrhotic patients with high liver cell proliferative activity. Hepatology. 2001;34:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Trerè D, Borzio M, Morabito A, Borzio F, Roncalli M, Derenzini M. Nucleolar hypertrophy correlates with hepatocellular carcinoma development in cirrhosis due to HBV infection. Hepatology. 2003;37:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Edited by Wang XL and Xu FM