Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2628
Revised: August 23, 2003
Accepted: December 6, 2003
Published online: September 15, 2004
AIM: To explore the effects of mifepristone, a progesterone receptor (PR) antagonist, on the proliferation of human gastric adenocarcinoma cell line SGC-7901 in vitro and the possible mechanisms involved.
METHODS: In situ hybridization was used to detect the expression of PR mRNA in SGC-7901 cells. After treatment with various concentrations of mifepristone (2.5, 5, 10, 20 μmol/L) at various time intervals, the ultrastructural changes, cell proliferation, cell-cycle phase distribution, and the expression of caspase-3 and Bcl-XL were analyzed using transmission electron microscopy (TEM), tetrazolium blue(MTT) assay, 3H-TdR incorporation, flow cytometry, and reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS: Mifepristone markedly induced apoptosis and inhibited cell proliferation of PR- positive SGC-7901 cells revealed by TEM, MTT assay and 3H-TdR incorporation, in a dose- and time-dependent manner. The inhibitory rate was increased from 8.98% to 51.29%. Flow cytometric analysis showed mifepristone dose-dependently decreased cells in S and G2/M phases, increased cells in G0/G1 phase, reduced the proliferative index from 57.75% to 22.83%. In addition, mifepristone up-regulated the expression of caspase-3, and down- regulated the Bcl-XL expression, dose-dependently.
CONCLUSION: Mifepristone effectively inhibited the proliferation of PR-positive human gastric adenocarcinoma cell line SGC-7901 in vitro through multiple mechanisms, and may be a beneficial agent against human adenocarcinoma.
-
Citation: Li DQ, Wang ZB, Bai J, Zhao J, Wang Y, Hu K, Du YH. Effects of mifepristone on proliferation of human gastric adenocarcinoma cell line SGC-7901
in vitro . World J Gastroenterol 2004; 10(18): 2628-2631 - URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2628
Gastric adenocarcinoma is the second most common cancer with the second highest mortality rate[1,2]. Presently, there is still no effective treatment means for patients with advanced gastric adenocarcinoma[3,4]. Chemotherapy or radiation therapy has generally shown some clinical response but little survival advantage and is not tolerated in many patients[5,6]. Therefore, there is a need to identify other therapeutic agents against the tumor.
Mifepristone is a progesterone receptor (PR) antagonist that has been widely used as the first-line drug for the termination of early pregnancy[7]. Interestingly, recent studies have proved that mifepristone could effectively inhibit the proliferation of PR-positive breast cancer[8-10], ovarian cancer[11,12], endometrial cancer[13], and prostate cancer[14] cells without serious side effects and drug resistance. However, the effects of mifepristone on gastric adenocarcinoma are still unknown. Therefore, the present study was undertaken to explore the effects of mifepristone on the proliferation of human gastric adenocarcinoma cell line SGC-7901 in vitro. Results showed that mifepristone effectively inhibited the proliferation of cultured SGC-7901 cells in vitro through multiple mechanisms.
Human gastric adenocarcinoma cell line SGC-7901, obtained from Wuhan University Type Culture Collection (Wuhan, China), was routinely maintained in phenol red-free RPMI1640 (Gibco BRL, Grand Island, NY) containing 100 mL/L fetal bovine serum (Hyclone, Logan, UT), 105 U/L penicillin and 100 mg/L streptomycin at 37 °C in a humidified atmosphere with 50 mL/L CO2 in air. When cells were grown to approximately 50% confluence, medium was replaced with serum-free RPMI1640. After 24 h, fresh media containing 2.5, 5, 10, 20 μmol/L mifepristone (Sigma Chemical Co., St Louis, MO) were added, respectively. Control cells were treated with the same volumes of vehicle (ethanol). Unless otherwise indicated, the cells were harvested after 96 h of incubation.
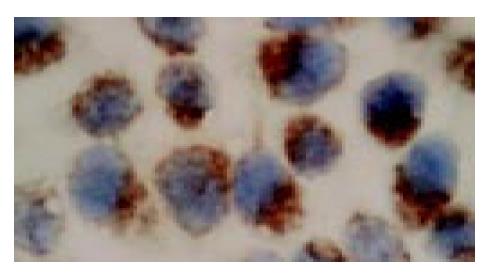
The expression of PR mRNA in SGC-7901 cells was detected by in situ hybridization (ISH) using an ISH detection kit for PR(Boster, Wuhan, China) according to the manufacturer’s instructions. Unless otherwise stated, all steps were performed at room temperature. Briefly, after 24 h of culture on the RNAse-free slides, cells were washed 3 times with phosphate buffered saline (PBS, pH7.4), fixed with 40 g/L paraformaldehyde in PBS containing 0.1 g/L diethylpyrocarbonate (DEPC-water) for 30 min, washed 3 times with 0.01 mol/L PBS, and then incubated with 5 mL/L hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity. After being rinsed with 0.01 mol/L PBS, cells were digested with proteinase K (10 g/mL in 0.01 mol/L PBS) at 37 °C for 15 min. Further washes with 0.5 mol/L PBS were performed before pre-hybridization for 3 h at 37 °C in the pre-hybridization solutions in a humidified environment. Hybridization was then performed with digoxigenin-labeled cRNA antisense probe (5 g/mL) overnight at 37 °C in a moist chamber. Subsequently cells were washed for 10 min with 2 SSC (1 SSC = 150 mmol/L NaCl, 15 mmol/L sodium citrate, pH7.0), followed by 0.5 SSC for 15 min, and finally 0.2 SSC for 15 min. After treatment with blocking reagent for 30 min, cells were incubated with biotin-labeled mouse anti-digoxigenin antibody at 37 °C for 1 h, washed 4 times, for 5 min each time, with 0.5 mol/L PBS, and then treated with SABC solutions at 37 °C for 20 min. Then cells were washed 3 times, for 5 min each time, with 0.5 mol/L PBS, incubated with biotin-labeled peroxidase (POD) at 37 °C for 20 min, washed three time with 0.5 mol/L PBS. Finally, cells were visualized with 3,3-diaminobezidine (DAB), counterstained with hematoxylin, dehydrated, cleared, mounted with neutralgum, and examined under an microscope. Brown-yellow deposits indicated the sites of hybridization. PR-positive breast cancer tissues were used as positive control, and probes were replaced by PBS as negative control.
Harvested cells were washed 3 times with PBS, fixed for 2 h with 2.5 g/L glutaraldehyde in PBS, and then post-fixed for 2 h at 4 °C with 1 g/L OsO4 in PBS. Cells were dehydrated using gradually increasing concentrations of ethanol from 50% to 100%, and then embedded in Epon 812. The ultra-thin sections (60 nm) were stained with uranyl acetate and lead citrate prior to examination at 50 kV with a Hitachi 600 transmission electron microscrope (Hitachi Corp., Tokyo, Japan).
SGC-7901 cells were seeded into 96-well plates at a density of 1 × 105/mL in RPMI1640. After 96 h of incubation with various concentrations of mifepristone, cell proliferation was measured by MTT (Sigma) reduction assay as described previously[15]. Absorbance at 570 nm (A570nm) was assayed. The inhibitory rate (IR) of SGC-7901 cells was calculated according to the equation as following: IR (%) = (A570nm in control group - A570nm in mifepristone-treated group)/A570nm in control group × 100%.
Cells were incubated at various time intervals without or with various concentrations of mifepristone, followed by treatment with 10 μCi 3H-TdR (Amersham, Arlington Heights, IL) for an additional 6 h. Then, cells were washed twice with 100 mL/L trichloroacetic acid by centrifugation and resuspension, and were continuously incubated for 30 min at 60 °C with 0.5 mL of NaOH (0.3 mol/L). Finally, the cell lysates were collected, and the radioactivity was measured by a liquid scintillation counter(Beckman LS1 801, USA).
The harvested cells were fixed with 700 mL/L ethanol at -20 °C for 30 min, and then stained with propidium iodide (Sigma) for 30 min in the dark. The stained cells were analyzed in a FACS Calibur flow cytometer (Becton Dickinson Labware, Lincoln Park, NJ) with excitation wavelength of 488 nm. The resulting histograms were analyzed by program MODFIT for cell distribution in cell cycle phase. Proliferative index (PI) was calculated according to the formula: PI (%) = (S + G2/M)/(G0/G1 + S + G2/M) × 100%.
Total proteins were extracted from harvested cells as described previously[16], and protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). An equal amount of cellular protein from extract of each group was added to a final volume of 100 μL of reaction mixture containing 0.2 mmol/L of a colorimetric caspase-3 substrate, acetyl-Asp-Glu-Val-Asp-ρ-nitroaniline (Ac-DEVD-ρNA; Calbiochem, San Diego, CA), followed by incubation at 30 °C for 10 min. Free ρ-nitroaniline (ρNA) released upon enzymatic cleavage was detected at 405 nm using a microplate reader (Bio-Rad). Caspase-3 activity correlated with the concentration of free ρNA generated in the reaction. Purified caspase-3 (Calbiochem) was used as positive control, whereas caspase-3 inhibitor I (Ac-DEVD-CHO, Calbiochem) was used as negative control.
Total RNA was extracted from the cells using TRIzol reagent(Gibco BRL) according to the manufacturer’s protocol. Two milligrams of total RNA were used for reverse transcription in a total volume of 20 μL with the SuperScript preamplification system (Promega, Madison, MI). Aliquots of 2 μL cDNA were subsequently amplified in a total volume of 50 μL using the GeneAmp PCR kit (Promega) following conditions recommended by the manufacturer. The sense and antisense primers for Bcl-XL were 5’-AGGCAGGCGATGAGTTTGAAC-3’ and 5’-GAACCACACCAGCCACAGTCA-3’, respectively. The sense and antisense primers for -actin used as an internal control were 5’-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3’ and 5’-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3’, respectively. The cycling conditions were 94 °C for 2 min, followed by 30 cycles of 92 °C for 30 s, at 62 °C for 30 s, and at 72 °C for 1 min and a final extension of 72 °C for 5 min. PCR products were separated on the 15 g/L agarose gel stained with ethidium bromide (EB) and viewed under ultraviolet light.
Data were expressed as mean ± SD. Statistical analysis was performed using the Student’s t test and the chi-square test. P < 0.05 was considered statistically significant.
In situ hybridization analysis showed that PR mRNA was highly expressed in the cultured SGC-7901 cells, which was mainly localized in the cytoplasm of the cells (Figure 1).
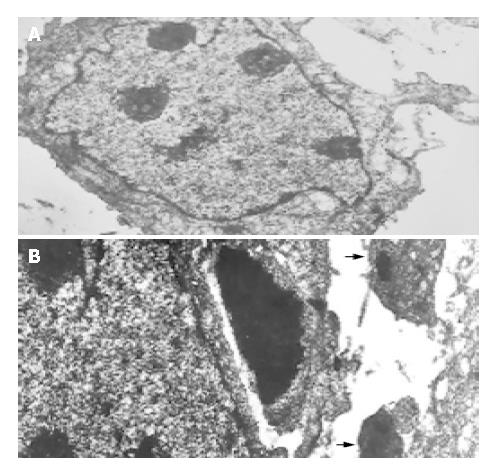
To assess the effect of mifepristone on the ultrastructural changes of SGC-7901 cells, transmission electron microscopic analysis was performed. Results revealed that mifepristone dose-dependently induced apoptosis, which was especially remarkable at the 20 μmol/L concentration (Figure 2B). However, the irregular and enlarged nuclear, multiple nucleoli and increased nucleus-to-cytoplasm ratio were clearly seen in the cells of control group (Figure 2A).
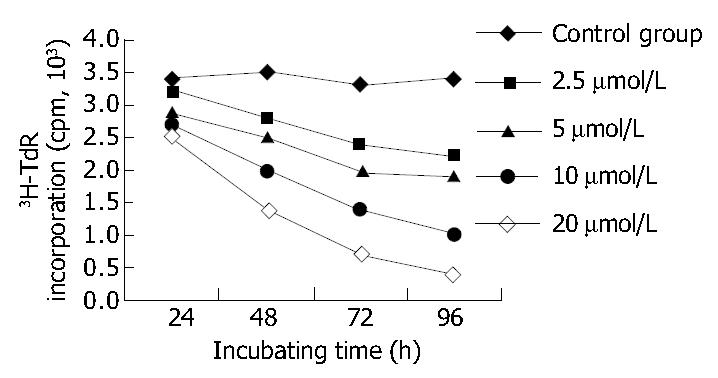
After 96 h of incubation with 2.5, 5, 10, 20 μmol/L mifepristone, MTT assay revealed A570nm was markedly decreased in a dose-dependent manner, and the inhibitory rate (IR) of SGC-7901 cells by mifepristone was 8.98%, 20.36%, 39.73% and 51.29%, respectively (Table 1). Figure 3 shows that 3H-TdR incorporation into DNA of SGC-7901 cells was significantly decreased in a dose- and time-dependent manner.
| Concentration (μmol/L) | A570nm (MTT) | Cell cycle phase distribution (%) | Caspase-3 activity (U) | ||
| G0/G1 | S | G2/M | |||
| 0 | 1.125 ± 0.048 | 42.25 ± 4.20 | 35.68 ± 3.98 | 22.07 ± 3.01 | 1.28 ± 0.28 |
| 2.5 | 1.024 ± 0.030 | 49.47 ± 5.68 | 30.82 ± 4.36 | 19.71 ± 2.41 | 2.79 ± 0.36 |
| 5 | 0.896 ± 0.035 | 52.23 ± 6.22 | 28.68 ± 3.64 | 19.01 ± 1.36 | 5.04 ± 0.29 |
| 10 | 0.678 ± 0.026 | 65.80 ± 5.63 | 25.93 ± 3.01 | 8.27 ± 1.10 | 9.46 ± 0.20 |
| 20 | 0.548 ± 0.031 | 77.16 ± 8.25 | 15.54 ± 2.54 | 7.29 ± 0.82 | 15.23 ± 0.41 |
The effect of mifepristone on the cell-cycle phase distribution of SGC-7901 cells was determined by flow cytometry. After treatment with mifepristone, there was a strong dose- dependent decrease in the percentage of S- and G2/M-phase cells, and with a concomitant increase in the percentage of cells in the G0/G1 phases of the cell cycle (Table 1). Additionally, there is a significant decrease in the proliferative index (PI) of the mifepristone-treated cells (50.53%, 47.69%, 34.20% and 22.83%) as compared with control group (57.75%, P < 0.01).
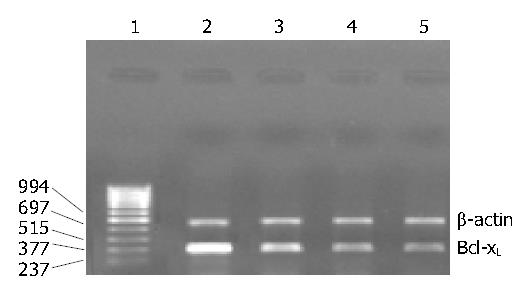
As shown in Table 1, mifepristone significantly up-regulated the activity of caspase-3 as compared with that in control group. Figure 4 shows the results of RT-PCR analysis for Bcl-xL mRNA expression. Results indicated that mifepristone dose-dependently inhibited the expression of Bcl-XL in the SGC-7901 cells.
Accumulating evidence demonstrates that PR level is closely associated with proliferation, invasion and metastasis of human gastric adenocarcinoma, as well as prognosis of patients[17-19]. Therefore, there has been increasing interest in the development of antiprogestins for tumor treatment. Mifepristone has been proved to be a potent and effective PR antagonist by competing with progesterone for PR binding, followed by binding to progesterone response element (PRE)[20]. In the present study, we proved that mifepristone effectively inhibited the proliferation of human gastric adenocarcinoma cell line SGC-7901 in vitro through induction of apoptosis and arresting the cell cycle progession.
Previous studies[21] found that the determination of PR levels was primarily used as a marker of a tumor’s responsiveness to mifepristone. To determine the expression of PR mRNA in the SGC-7901 cells, in situ hybridization was performed. We found that PR mRNA was highly expressed in cultured SGC-7901 cells. Meanwhile, the result is in agreement with the work of Cui et al[22], who reported that the concentrations of PR protein in the cytoplasm and nuclei of cultured SGC-7901 cells were 20.3 fmoL/mg and 22.7 fmoL/mg, respectively, revealed by dextran-coated charcoal (DCC) assay. Thus, we speculate that the growth inhibitory effects of mifepristone in our study might be mediated, at least in part, by PR.
In our study, TEM, MTT assay and 3H-TdR incorporation were used to evaluate the effect of mifepristone on the proliferation of SGC-7901 cells in vitro. We found that mifepristone exerted significantly anti-proliferative effect on cultured SGC-7901 cells in vitro in a dose- and time-dependent manner. The results are in agreement with those of previous studies on other tumor cell lines in vitro[9-11,23].
Although a number of studies have proved that mifepristone has the growth inhibitory effects on tumor cells, but the mechanisms remain unknown. These theoretically could be related with the changes of the dynamics of cell proliferation. The hypothesis was demonstrated by the work of Thomas et al[24], who proved that mifepristone inhibited the proliferation of the MCF-7 human breast cancer cells by arresting them in the G0/G1 phase of the cell cycle. Recently, Peters et al[25] reported that mifepristone up-regualted the expression of cell cycle protein p21WAF/cip1 in medroxyprogesterone acetate-induced ductal mammary adenocarcinoma. Thus, we further explored the effects of mifepristone on the cell cycle of SGC-7901 cell using flow cytometry. Results showed that mifepristone markedly increased the proportion of cells in G0/G1, and simultaneously decreased the percentage of cells in S- and G2/M-phase. Taken together, it seems reasonable to conclude that the growth inhibitory effects of mifepristone on SGC-7901 cells is partially due to an accumulation of cells in G0/G1 phase.
To explore whether or not the apoptosis-related genes contributed to the inhibitory effect of mifepristone on the SGC-7901 cells, we assessed the activity of caspase-3, an executioner of apoptosis[26,27], and mRNA level of Bcl-XL, an anti-apoptotic gene. Our findings showed that mifepristone dose-dependently up-regulated caspase-3 activity and down-regulated Bcl-XL mRNA expression. This result is supported by previous studies on prostate cells. El Etreby et al[14] reported that mifepristone significantly induced apoptosis in LNCaP prostate cancer cells in a time- and dose-dependent manner through down-regulation of Bcl-2 protein and induction of caspase-3 activity. Collectively, it is possible that the activation of caspase-3 and the degradation of Bcl-XL are partially responsible for the antiproliferative effects of mifepristone on cultured SGC-7901 cells.
In conclusion, the study demonstrated that mifepristone exerted marked antiproliferative effect on the PR-positive SGC-7901 cells by inducing apoptosis, arresting cell cycle progression, up-regulating caspase-3 activity and down-regulating Bcl-XL mRNA expression. These results indicate that mifepristone may be a useful agent against human gastric adenocarcinoma although further studies are clearly needed to prove the possibility.
| 1. | Albert C. Clinical aspects of gastric cancer. In: Rustgi AK, editors. Gastrointestinal cancer: biology, diagnosis and therapy. Philadelphia: Lippincott Raven. 1995;197-216. |
| 2. | Lu JB, Sun XB, Dai DX, Zhu SK, Chang QL, Liu SZ, Duan WJ. Epidemiology of gastroenterologic cancer in Henan Province, China. World J Gastroenterol. 2003;9:2400-2403. [PubMed] |
| 3. | Maehara Y, Kakeji Y, Masuda T, Sakoguchi T, Imamura M, Ohgaki K, Taniguchi K, Sakurai M, Futatsugi M, Kimura Y. [Treatment of gastric cancer: current state and future prospect]. Fukuoka Igaku Zasshi. 2003;94:285-295. [PubMed] |
| 4. | De Paoli A, Buonadonna A, Boz G, Lombardi D, Innocente R, Tumolo S, Tosolini G, Rossi C, Trovò MG, Frustaci S. Combined modality treatment for locally advanced gastric cancer. Suppl Tumori. 2003;2:S58-S62. [PubMed] |
| 5. | Macdonald JS. Chemotherapy in the management of gastric cancer. J Clin Oncol. 2003;21:276s-279s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Valentini V, Cellini F, D'Angelillo RM. Combined treatments in gastric cancer: radiotherapy. Suppl Tumori. 2003;2:S39-S44. [PubMed] |
| 7. | Mahajan DK, London SN. Mifepristone (RU486): a review. Fertil Steril. 1997;68:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Liang Y, Hou M, Kallab AM, Barrett JT, El Etreby F, Schoenlein PV. Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta1. Int J Oncol. 2003;23:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | El Etreby MF, Liang Y, Wrenn RW, Schoenlein PV. Additive effect of mifepristone and tamoxifen on apoptotic pathways in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1998;51:149-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | El Etreby MF, Liang Y. Effect of antiprogestins and tamoxifen on growth inhibition of MCF-7 human breast cancer cells in nude mice. Breast Cancer Res Treat. 1998;49:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Rose FV, Barnea ER. Response of human ovarian carcinoma cell lines to antiprogestin mifepristone. Oncogene. 1996;12:999-1003. [PubMed] |
| 12. | Rocereto TF, Saul HM, Aikins JA, Paulson J. Phase II study of mifepristone (RU486) in refractory ovarian cancer. Gynecol Oncol. 2000;77:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Schneider CC, Gibb RK, Taylor DD, Wan T, Gerçel-Taylor C. Inhibition of endometrial cancer cell lines by mifepristone (RU 486). J Soc Gynecol Investig. 1998;5:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | El Etreby MF, Liang Y, Johnson MH, Lewis RW. Antitumor activity of mifepristone in the human LNCaP, LNCaP-C4, and LNCaP-C4-2 prostate cancer models in nude mice. Prostate. 2000;42:99-106. [PubMed] [DOI] [Full Text] |
| 15. | Saikawa Y, Kubota T, Furukawa T, Suto A, Watanabe M, Kumai K, Ishibiki K, Kitajima M. Single-cell suspension assay with an MTT end point is useful for evaluating the optimal adjuvant chemotherapy for advanced gastric cancer. Jpn J Cancer Res. 1994;85:762-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Sridhar S, Ali AA, Liang Y, El Etreby MF, Lewis RW, Kumar MV. Differential expression of members of the tumor necrosis factor alpha-related apoptosis-inducing ligand pathway in prostate cancer cells. Cancer Res. 2001;61:7179-7183. [PubMed] |
| 17. | Matsui M, Kojima O, Kawakami S, Uehara Y, Takahashi T. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today. 1992;22:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 18. | Oshima CT, Wonraht DR, Catarino RM, Mattos D, Forones NM. Estrogen and progesterone receptors in gastric and colorectal cancer. Hepatogastroenterology. 1999;46:3155-3158. [PubMed] |
| 19. | Korenaga D, Orita H, Okuyama T, Kinoshita J, Maekawa S, Ikeda T, Sugimachi K. Sex hormone-receptor-negative tumors have a higher proliferative activity than sex hormone-receptor-positive tumors in human adenocarcinomas of the gastrointestinal tract. Surg Today. 1998;28:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Meyer ME, Pornon A, Ji JW, Bocquel MT, Chambon P, Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990;9:3923-3932. [PubMed] |
| 21. | Lin VC, Aw SE, Ng EH, Ng EH, Tan MG. Demonstration of mixed properties of RU486 in progesterone receptor (PR)-transfected MDA-MB-231 cells: a model for studying the functions of progesterone analogues. Br J Cancer. 2001;85:1978-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Cui H, Lu P, Yu QS, Chen ZH. Sex hormone receptor in the cytoplasm and nuclear of human gastric cancer cell line SGC-7901. Zhongliu Fangzhi Yanjiu. 1998;25:9-10. |
| 23. | Yokoyama Y, Shinohara A, Takahashi Y, Wan X, Takahashi S, Niwa K, Tamaya T. Synergistic effects of danazol and mifepristone on the cytotoxicity of UCN-01 in hormone-responsive breast cancer cells. Anticancer Res. 2000;20:3131-3135. [PubMed] |
| 24. | Thomas M, Monet JD. Combined effects of RU486 and tamoxifen on the growth and cell cycle phases of the MCF-7 cell line. J Clin Endocrinol Metab. 1992;75:865-870. [PubMed] |
| 25. | Peters MG, Vanzulli S, Elizalde PV, Charreau EH, Goin MM. Effects of antiprogestins RU486 and ZK98299 on the expression of cell cycle proteins of a medroxyprogesterone acetate (MPA)-induced murine mammary tumor. Oncol Rep. 2001;8:445-449. [PubMed] |
| 26. | Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3353] [Cited by in RCA: 3512] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 27. | Yang X, Stennicke HR, Wang B, Green DR, Jänicke RU, Srinivasan A, Seth P, Salvesen GS, Froelich CJ. Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J Biol Chem. 1998;273:34278-34283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
Edited by Xu FM Proofread by Zhu LH