Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2415
Revised: March 22, 2004
Accepted: April 9, 2004
Published online: August 15, 2004
AIM: It is reasonable to assume that microchimerism could also be involved in the induction of primary biliary cirrhosis (PBC). However, previous reports investigated only fetus-microchimerism in women patients. Maternal microchimerism has not been investigated until now. The current study aimed to clear either maternal microchimerism was involved in the pathogenesis of PBC or not.
METHODS: We used fluorescence in situ hybridization on paraffin-embedded tissue (We called “Tissue-FISH”.) to determine whether maternal cells infiltrated in male patients who were diagnosed as having PBC. Tissue-FISH was performed by using both X and Y specific probes on the biopsy liver sample of 3 male PBC patients.
RESULTS: Infiltrating lymphocytes demonstrated both X and Y signals in all 3 male patients.
CONCLUSION: Maternal microchimerism dose not play a significant role in PBC. PBC may not relate to fetus and maternal microchimerism.
- Citation: Nomura K, Sumida Y, Yoh T, Morita A, Matsumoto Y, Taji S, Yoshida N, Minami M, Itoh Y, Horiike S, Kataoka K, Taniwaki M, Okanoue T. Lack of evidence for leukocyte maternal microchimerism in primary biliary cirrhosis. World J Gastroenterol 2004; 10(16): 2415-2416
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2415
Molecular biological techniques have shown that fetal cells can be detected in maternal peripheral blood in most pregnancies[1-3]. On this observation, the radical hypothesis of autoimmune disease, proposed in 1996, may be caused by fetal cells that are retained after pregnancies[4]. In systemic sclerosis, it was reported that fetal antimaternal graft-versus-host reactions might be involved in the pathogenesis of systemic sclerosis in some women[5,6]. Thus, the hypothesis that microchimerism might cause the primary biliary cirrhosis (PBC) is very fascinating[7], because it may explain clinical similarities between PBC and chronic graft-versus-host disease (GVHD) in liver after bone marrow transplantation. Some previous reports hypothesized that fetal microchimerism may also play a role in the pathogenesis of PBC[8-11]. However, they concluded that fetal microchimerism was not important in the development of PBC.
Although there is a possibility that PBC may be developed by maternal microchimerism, not fetal microchimerism in female patients, maternal microchimerism has not been considered until now. Thus, we produced one hypothesis that maternal microchimerism might cause PBC.
To test the hypothesis, we investigated 3 male PBC patients by fluorescence in situ hybridization (FISH) on paraffin-embedded tissues.
Three male patients with PBC were studied after giving informed consent. They were diagnosed at Kyoto Prefectural University of Medicine by liver biopsy and the positivity of serum antimitochondrial antibody (AMA). All patients were compatible with the feature of PBC. None of these patients had scleroderma. They had no experience of blood transfusion.
We reported tissue-FISH method previously[12-14]. Sections from paraffin-embedded tissues were placed on silane-coated glass slides. The slides were deparaffinized immediately in 2 rinses of 1000 g/L xylene for 10 min each. Each slide was rehydrated in an ethanol series for 5 min. The slides were then treated with 0.2 mol/L HCl for 20 min, followed by 2 × SSC (0.3 mol/L sodium chloride and 0.03 mol/L sodium citrate) for 20 min at 80 °C, treated with 0.05 mg/mL proteinase K in TEN (0.05 mol/L Tris-HCl, pH 7.8, 0.01 mol/L EDTA, and 0.01 mol/L sodium chloride) for 10 min at 37 °C, and placed in 40 g/L formaldehyde in PBS for 10 min. Both FISH probes and target DNA were denatured simultaneously for 10 min at 90 °C, and the slides were incubated overnight at 42 °C, placed in 2 × SSC for 10 min at 42 °C, washed twice in 2 × SSC/500 g/L formaldehyde formamide for 5 min each at 42 °C, washed 2 × SSC for 5 min at 42 °C, and counterstained in 2 × SSC/0.03 μg/mL DAPI.
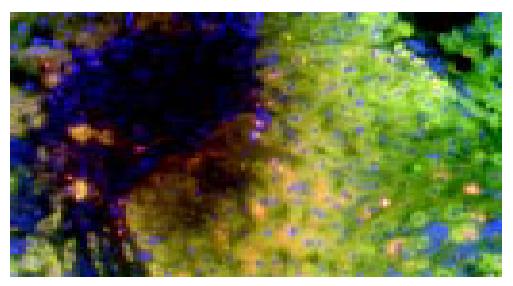
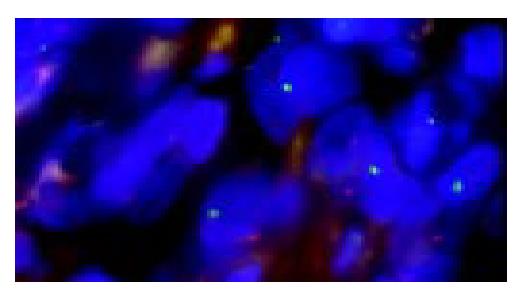
It was easy to recognize the architecture of liver tissues by fluorescence microscope BX40-RF (Olympus, Tokyo, Japan). The lymphocytes aggregated at the portal zone (Figure 1). We assessed only non-overlapping nuclei having XY or XX signals. Almost infiltrating lymphocytes at septa revealed the XY chromosome pattern (Figure 2). The frequency of nuclei having XY in 3 patients is 96%, 98%, and 97%, respectively. (P < 0.01).
The hypothesis that fetal microchimerism causing PBC is fascinating. However, this hypothesis does not explain why some cases with PBC were fetal microchimerism negative and why even males and nulliparous women were suffering from PBC. On the other hand, the hypothesis that maternal microchimerism causes PBC is more fascinating, because it resolves the above questions. Every one has the risk of maternal microchimerism, because it has been already established that maternal cells persist for a long-term in immunocompetent offsprings[15]. However, this hypothesis could not be resolved by polymerase chain reaction (PCR), because PCR can only detect the Y-chromosome specific DNA.
To clear whether infiltrating lymphocytes derived from either maternal or fetal cells in male PBC patients, we performed tissue-FISH. Tissue-FISH is able to distinguish infiltrating lymphocytes from hepatocytes, because this method preserves the architecture of liver tissues and the shapes of cells. Tissue-FISH revealed that almost infiltrating lymphocytes were male cells in the liver sample of male patients. These results showed that maternal microchimerism did not play an important role in introducing and maintaining primary biliary cirrhosis.
In conclusion, we were unable to provide the evidence of maternal microchimerism in PBC patients. Although PBC shows the similar histological features with GVHD, PBC may be different from GVHD in etiology.
| 1. | Lo YM, Patel P, Sampietro M, Gillmer MD, Fleming KA, Wainscoat JS. Detection of single-copy fetal DNA sequence from maternal blood. Lancet. 1990;335:1463-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Thomas MR, Williamson R, Craft I, Yazdani N, Rodeck CH. Y chromosome sequence DNA amplified from peripheral blood of women in early pregnancy. Lancet. 1994;343:413-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Bianchi DW. Prenatal diagnosis by analysis of fetal cells in maternal blood. J Pediatr. 1995;127:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Nelson JL. Maternal-fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Artlett CM, Smith JB, Jimenez SA. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998;338:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 307] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, Bean MA, Ober C, Bianchi DW. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 412] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | McDonnell WM. Is primary biliary cirrhosis a complication of pregnancy? Hepatology. 1998;28:593-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Tanaka A, Lindor K, Gish R, Batts K, Shiratori Y, Omata M, Nelson JL, Ansari A, Coppel R, Newsome M. Fetal microchimerism alone does not contribute to the induction of primary biliary cirrhosis. Hepatology. 1999;30:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Rubbia-Brandt L, Philippeaux MM, Chavez S, Mentha G, Borisch B, Hadengue A. FISH for Y chromosome in women with primary biliary cirrhosis: lack of evidence for leukocyte microchimerism. Hepatology. 1999;30:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Invernizzi P, De Andreis C, Sirchia SM, Battezzati PM, Zuin M, Rossella F, Perego F, Bignotto M, Simoni G, Podda M. Blood fetal microchimerism in primary biliary cirrhosis. Clin Exp Immunol. 2000;122:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Corpechot C, Barbu V, Chazouillères O, Poupon R. Fetal microchimerism in primary biliary cirrhosis. J Hepatol. 2000;33:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Nomura K, Sekoguchi S, Ueda K, Nakao M, Akano Y, Fujita Y, Yamashita Y, Horiike S, Nishida K, Nakamura S. Differentiation of follicular from mucosa-associated lymphoid tissue lymphoma by detection of t(14; 18) on single-cell preparations and paraffin-embedded sections. Genes Chromosomes Cancer. 2002;33:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Nomura K, Yoshino T, Nakamura S, Akano Y, Tagawa H, Nishida K, Seto M, Nakamura S, Ueda R, Yamagishi H. Detection of t(11; 18)(q21; q21) in marginal zone lymphoma of mucosa-associated lymphocytic tissue type on paraffin-embedded tissue sections by using fluorescence in situ hybridization. Cancer Genet Cytogenet. 2003;140:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Matsumoto Y, Nomura K, Matsumoto S, Ueda K, Nakao M, Nishida K, Sakabe H, Yokota S, Horiike S, Nakamine H. Detection of t(14; 18) in follicular lymphoma by dual-color fluorescence in situ hybridization on paraffin-embedded tissue sections. Cancer Genet Cytogenet. 2004;150:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 339] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Chen WW and Xu FM