Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2417
Revised: February 2, 2004
Accepted: February 24, 2004
Published online: August 15, 2004
AIM: Gastrointestinal stromal tumor (GIST) is a rare type of cancer. Computed tomography (CT) is an imaging modality of choice for diagnosing GIST. The aim of this retrospective study was to review the CT imaging features of 17 GIST patients.
METHODS: From 1995 to 2003, there were 47 patients with pathologically proven GISTs at our hospital. Of these, 17 patients underwent preoperative CT. We collected and analyzed these CT images. The CT imaging features included tumor diameter, number and location, tumor margin, location of metastasis, hounsfield units of tumor and effect of contrasts. In addition, we also recorded the surgical findings, including complications, tumor size and location for comparative analysis.
RESULTS: The results showed that 12 (70%) tumors were located in the stomach and five (30%) were located in the jejunum mesentery. GISTs were extraluminal in 12 (70%) patients. The tumor margins of 13 (76%) tumors were well defined and irregular in four (24%). The effect of contrast enhancement on GIST CT imaging was homogenous enhancement in 13 (76%) and heterogeneous enhancement in four (24%). The hounsfield units (HU) were 30.41 ± 5.01 for precontrast images and postcontrast hounsfield units were 51.80 ± 9.24.
CONCLUSION: The stomach was the commonest site of GIST occurrence among our patients. The CT features of GIST were well-defined tumor margins, homogenous enhancement on postcontrast CT images.
- Citation: Lee CM, Chen HC, Leung TK, Chen YY. Gastrointestinal stromal tumor: Computed tomographic features. World J Gastroenterol 2004; 10(16): 2417-2418
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2417.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2417
The term gastrointestinal stromal tumor (GIST) has traditionally been used as a descriptive term for soft tissue tumors of the gastrointestinal tract. Although their exact incidence is still somewhat unclear, it is now estimated that between 5000 and 10000 people each year develop GISTs in the world; men and women are equally affected[1]. GISTs were previously thought to be smooth muscle neoplasms, and most were classified as leiomyosarcoma. With the advent of immunohistochemistry and electron microscopy, it has become apparent that GIST may have myogenic features (smooth muscle GIST), neural attributes (gastrointestinal autonomic nerve tumor), characteristics of both muscle and nerve (mixed GIST) or may lack differentiation (GIST not otherwise specified)[2]. GISTs are often discovered incidentally at surgery and should be completely excised. The increasing use of computed tomography (CT) and endoscopy of the upper gastrointestinal tract is a non-or minimally invasive means for the detection of asymptomatic GISTs[3].
In this retrospective study, we analyzed our experience with 17 patients with GISTs who were presurgically investigated by using CT and described the anatomic distribution and imaging features of GIST.
From 1995 to 2003, there were 47 patients with pathologically proven GISTs at Taipei Medical University Hospital (TMUH) and Wan Fang Hospital (WFH). Of these, 17 (8 males, 9 females, with ages ranging from 33 to 91 years, mean age: 64 years) underwent preoperative CT. We collected and analyzed these CT images.
The abdominopelvic CT scans (HiSpeed CT/I; GE Medical Systems, Milwaukee, WI, USA) were typically obtained after oral administration of 1000 mL 40 g/L iothalamate meglumine (Mallinckrodt, USA) and intravenous administration of 100 mL (350 mg/mL) iohexol (Nycoveien, Norway) at a flow rate of 2 mL/s, with a section thickness of 10 mm and a pitch of 1.5. The CT imaging features included tumor diameter, number and location, tumor margin (well defined, irregular or clearly invasive), location of metastasis, hounsfield units of tumor and effect of contrast. These characteristics were reviewed blindly by three radiology diplomates. In addition, we also recorded the surgical findings, including complications, tumor size and location for comparison.
The CT imaging findings showed that 14 (82%) patients had one tumor and 3 (18%) patients had two tumors. GIST size ranged from 2 to 19 cm (5.4 ± 1.8 cm). Tumors were located in the stomach in 12 (70%) patients and 5 (30%) patients had tumor located in the jejunal mesentery. GISTs were extraluminal in 12 (70%) patients and intraluminal in 5 (30%). GISTs caused intraluminal bowel obstruction in two patients.
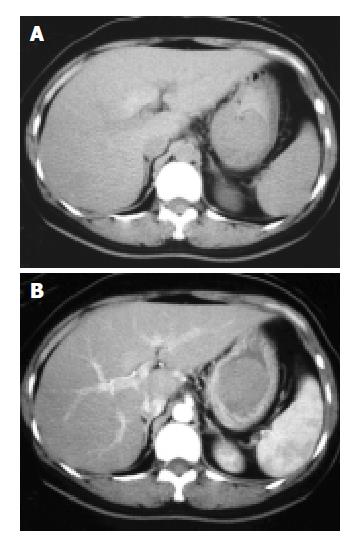
The mean precontrast hounsfield units were 30.41 ± 5.01 and the mean postcontrast hounsfield units were 51.80 ± 9.24. The effect of contrast enhancement on GIST CT imaging was slight enhancement (Figure 1). Thirteen (76%) showed homogenous enhancement and 4 (24%) showed heterogeneous enhancement.
Tumors were well defined in 13 (76%) patients and irregular in 4 (24%) patients. There was no clearly invasive or vascular encasement of tumors among our patients. Three (18%) patients had metastasis, two to the liver and one to the lung. Twelve patients underwent follow-up CT (range of follow-up period from date of diagnosis, 2-40 mo, mean, 22 mo). No patients relapsed.
Operative findings showed that 14 (82%) patients had one tumor and 3 (18%) patients had two tumors. The smallest GIST was 2 cm × 2 cm × 1.8 cm and the largest was 19 cm × 16 cm × 8.5 cm in size. The commonest complications among our patients were gastrointestinal tract chronic inflammation, diarrhea and wound infection. In addition, 17 patients all underwent lymphadenectomies but no metastasis to the lymph nodes was found.
The distribution of 725 malignant smooth muscle tumors of the gastrointestinal tract was 47.3% in the stomach, 35.4% in the small intestine, 4.6% in the colon and 7.4% in the rectum, according to Skandalak and Gray[4]. In the report by Akwari et al[5], 68.3% of GISTs were in the stomach, 25.4% were in the small intestine, 2.6% were in the colon and 3.7% were in the rectum. According to a previous study[6], GIST can also occur in the omental and mesenteric tissues, the duodenum and other sites of the gastrointestinal tract. In our study, 12 (70%) patients had tumors located in stomach and five (30%) patients had tumors located in the jejunal mesentery, a distribution more similar to that reported by Akwari et al[5].
According to our results, the precontrast hounsfield units of the tumors were 30.41 ± 5.01 and the postcontrast hounsfield units were 51.80 ± 9.24. The postcontrast hounsfield units were 70% higher than the precontrast hounsfield units. Suster[7] reported hounsfield units of 33.2 ± 1.25 on precontrast imaging and 55.32 ± 5.22 on postcontrast imaging, with 68% enhancement. Ludwig[8] reported hounsfield units of 34.21 ± 1. 33 on precontrast imaging and 56.29 ± 3.12 on postcontrast imaging, with 67% enhancement. We believe that precontrast hounsfield units of 30 to 35 and postcontrast hounsfield units of 50 to 60 are indicative of GISTs on CT.
We analyzed the correlation of contrast type and tumor size. Of the 17 patients, 4 (24%) had heterogeneous contrast enhancement and 13 (76%) had homogenous contrast enhancement. The mean tumor diameter of the heterogeneous tumors was 11.6 ± 2.1 cm and that of the homogenous tumors was 3.8 ± 1.3 cm. We found that large tumor sizes appeared to be related to heterogeneous enhancement. Our result is similar to that of Conlon et al[9]. In addition, we found tumors in 13 (76%) of our patients were well-defined, and in Lee’s study[10], more than two-thirds of patients also had well-defined GISTs. Thus, well-defined tumors appear to be a feature of GISTs on CT imaging.
Licht et al[11] proposed that the relationship between multiple tumors and metastasis needed further investigation. In our study, we had three patients with multiple tumors and also had three patients with metastases. Only one patient with liver metastasis had multiple tumors; the other two patients with metastasis had only single tumor. Our data appear to indicate that there is no evident correlation between multiple tumors and metastasis. Additionally, 3 (18%) of our patients had metastasis compared to other studies[12,13] in which 20% to 35% of patients had metastasis, but this difference was not statistically significant. Fong et al[13] reported that the metastasis percentage was related to the degree of lymph node involvement. Based on our surgical findings, all the patients who had metastasis had no lymph node involvement. Thus, our results differed from those reported by Fong.
In conclusion, the stomach was the commonest site of GIST tumor location among our patients, with a mean tumor diameter of 5.4 ± 1.8 cm. The CT features of GISTs included well-defined tumor margins and predominantly homogenous contrast enhancement, with precontrast hounsfield units of 30 to 35 and postcontrast hounsfield units of 50 to 60. According to the percentage presented above, we also found a “4 seventy rule” in our GIST images review: 70% tumors were located in the stomach, 70% tumors were extraluminal, 76% tumor margins were well defined, 76% GIST CT imagings were homogenous enhancement. In addition, metastasis was not related to the degree of lymph node involvement or tumor number in our study.
| 1. | Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 322] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 563] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 4. | SKANDALAKIS JE, GRAY SW. SMOOTH MUSCLE TUMORS OF THE ALIMENTARY TRACT. Prog Clin Cancer. 1965;10:692-708. [PubMed] |
| 5. | Akwari OE, Dozois RR, Weiland LH, Beahrs OH. Leiomyosarcoma of the small and large bowel. Cancer. 1978;42:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 659] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Suster S. Gastrointestinal stromal tumors. Semin Diagn Pathol. 1996;13:297-313. [PubMed] |
| 8. | Ludwig DJ, Traverso LW. Gut stromal tumors and their clinical behavior. Am J Surg. 1997;173:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol. 1995;2:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Lee YT. Leiomyosarcoma of the gastro-intestinal tract: general pattern of metastasis and recurrence. Cancer Treat Rev. 1983;10:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Licht JD, Weissmann LB, Antman K. Gastrointestinal sarcomas. Semin Oncol. 1988;15:181-188. [PubMed] |
| 12. | Lindsay PC, Ordonez N, Raaf JH. Gastric leiomyosarcoma: clinical and pathological review of fifty patients. J Surg Oncol. 1981;18:399-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 362] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Edited by Zhu LH Proofread by Xu FM