Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2327
Revised: January 2, 2004
Accepted: February 18, 2004
Published online: August 15, 2004
AIM: To find new serum biomarkers for liver cirrhosis (LC) in chronic carriers of hepatitis B virus (HBV).
METHODS: Surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF) mass spectrometry was used to discover biomarkers for differentiating HBV induced LC from non-cirrhotic cohorts. A training population of 25 patients with HBV-induced LC, 20 patients with HCC, and 25 closely age-matched healthy men, was studied.
RESULTS: Two biomarkers with Mr 7772 and 3933 were detected in sera of non-cirrhotic cohorts, but not in patients with HBV-induced LC. A sensitivity of 80% for all LC patients, a specificity of 81.8% for all non-cirrhotic cohorts and a positive predictive value of 75% for the study population were obtained.
CONCLUSION: These two serum biomarkers for HBV-induced LC might be used for diagnosis and assessment of disease progression.
- Citation: Zhu XD, Zhang WH, Li CL, Xu Y, Liang WJ, Tien P. New serum biomarkers for detection of HBV-induced liver cirrhosis using SELDI protein chip technology. World J Gastroenterol 2004; 10(16): 2327-2329
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2327
Liver cirrhosis (LC), the end-stage of liver fibrosis, is generally irreversible. Patients with LC caused by chronic infection of HBV are at high risks of hepatocellular carcinoma and high death rate[1,2]. Although some serum assays are on the way to differentiate chronic HBV infection or LC from HCC, pretreatment liver biopsy has been considered as the “gold standard” for assessing the grade of liver injury and stage of liver fibrosis. Clinicians relying on liver biopsy are able to correctly diagnose the stage of fibrosis or presence of cirrhosis in 80% patients[3]. However, liver biopsy can be associated with significant expense, manpower issues, and risk of patient injury. As a result, we still need to identify noninvasive tests that could replace liver biopsy.
Protein profiles might reflect the pathological state of HBV infection. The relationship between protein profile and disease progression could be achieved by analyzing the complex serum proteomic patterns[4,5]. We used a protein biochip surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF) mass spectrometry coupled with an artificial intelligence learning algorithm to differentiate HBV induced LC from non-cirrhosis cohorts. A blinded test was used to determine the sensitivity and specificity of the established pattern.
Of the 107 serum samples selected, 40 were from patients with HBV-induced LC and 30 from patients with HCC from You’an Hospital, Beijing, China, 37 from healthy men provided by Center of Cancer Prevention and Treatment, Zhongshan University, China. All HBV infected patients with LC were examined by ELISA and were HBeAg positive in serum. The final diagnoses were pathologically confirmed and specimens were obtained before treatment. All samples were fresh and stored at -70 °C and closely age-matched.
Three different chip chemistries (cationic, anionic, and Cu metal binding, Ciphergen Biosystems, Inc, Fremont, CA) were tested to determine which provided the best serum profiles in terms of number and resolution of protein peaks. It showed that WCX2 weak cationic chip gave the best result. A total of 10 μL of each sample was diluted into 20 μL with U9 buffer (1 × PBS, 9 mol/L urea, 1% CHAPS) and mixed. The mixing step was repeated several times on ice for a total of 30 min. An eight-spot WCX chip was washed with 50 mmol/L sodium acetate (pH 4.0) twice. Then sodium acetate buffer was added to U9-treated sample to make a further 1:13 dilution. The diluted serum mixture (100 μL) was applied to a protein chip array and incubated for 1 h on a shaker. After washing with the same sodium acetate buffer three times followed by a quick water rinse, 0.5 μL of saturated sinapinic acid (SPA) solution was applied onto each spot and allowed to air-dry. Then chips were performed on Protein Biological System II(c) mass spectrometer reader (PBSII, Ciphergen Biosystems, Inc).
Classification model was built up with Biomarker Pattern’s Software (BPS, Ciphergen Biosystems, Inc). Training data set consisted of 70 serum samples (25 from patients with LC, 20 from patients with liver cancer, and 25 from healthy individuals). A classification tree was set up to divide the data set into two bins based on the intensities of peaks. At each bin a peak intensity threshold was set. If the peak intensity of a sample was lower than or equal to the threshold, this sample would go to the left-side bin. Otherwise, the sample would go to the right-side bin. The process would go on until a blind sample entered a final bin, either labeled at Con (control sample) or LC (LC serum). Peaks selected by the process to form the model were the ones that yielded the least classification error when they were combined to use.
Data set from double-blind trials consisted of 37 serum samples (15 from patients with LC, 10 from patients with liver cancer, and 12 from healthy individuals) and was used to test the model.
Specificity and sensitivity were respectively calculated as the proportion of the number of non-cirrhotic samples correctly identified to the total number of non-cirrhotic samples. Positive predictive value gives the probability of disease if a test result is positive.
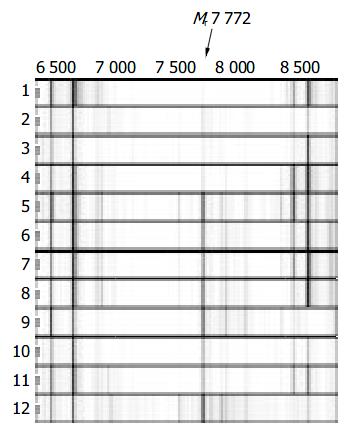
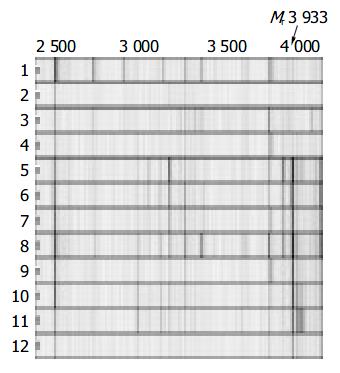
As various chip array chemistries provided different serum protein profiles in terms of number and resolution of protein peaks, WCX2, SAX2 and IMAC3-Cu metal binding chip arrays were tested, respectively. WCX2 binding chip was observed to give the best results. To demonstrate the reproducibility of the mass spectra, 8 independently obtained spectra of a serum sample of a healthy man were performed by between-run assay. We calculated that the coefficient of variance for seven selected M/Z peaks whose relative intensities were above 25 with the highest amplitude < 10%. As shown in Figure 1, serum spectra from patients and healthy men do not show large variations. Therefore, small variations between different sample groups could be used for biomarker discovery. SELDI-TOF spectra of randomly selected serum samples of patients with HBV induced LC, patients with HCC, and healthy individuals are shown in Figures 1 and 2. Two proteins of Mr 7 772 and 3 933 were down-regulated in LC or up-regulated in non-cirrhotic group (healthy/HCC).
Peak labeling was performed with Biomarker Wizard of Ciphergen ProteinChip software 3.1.1. The peak intensities were then transferred to Biomarker Pattern’s software. Totally 35 peaks from Mr 2000 to 30000 were selected to construct the classification model. Figure 3 shows the tree structure and sample distribution. Two peaks, Mr 7772 and 3933, were chosen to set up the decision tree, respectively. At Node 1, samples of Mr 7772 with peak intensities lower than or equal to 7.514 went to Terminal Node 1, which had 9 control samples and 21 LC samples. Otherwise, samples entered Node 2, which had 40 samples. At Node 2, samples of Mr 3933 with peak intensities lower than or equal to 8.217 went to Terminal Node 2, which had 1 control sample and 3 LC samples. The other samples entered Terminal Node 3, which had 35 control samples and l LC sample. The model identified 70 samples, 36 in control and 34 in LC, and yielded a sensitivity of 96% and specificity of 77.8%. When the double-blind sample data set was used to challenge the model, the model predicted a sensitivity of 80% and specificity of 81.8%. The positive predictive value was 75%.
HBV infection often leads to a prolonged active viral replication, HBV DNA integration and eventually LC[6]. About 55-85% of LC patients will develop hepatocellular carcinoma, which always has bad prognosis. It is estimated that HCC may be responsible for more than 1 million deaths annually and it is the fifth most frequent cause of cancer death worldwide[7]. Liver biopsy has remained the gold standard for identification of patients with liver diseases. However, the differential diagnosis between HCC and LC is sometimes difficult and new biochemical markers for HCC are required. In recent years, several non-invasive serum biomarkers have been considered to diagnose LC associated with HBV, including hyaluronic acid (HA)[8,9], type III procollagen peptide, laminin and type IV collagen[10].
Among the non-invasive serum biomarkers for liver fibrosis and cirrhosis, HA was reported to be the best marker for diagnosis[11]. HA with a molecular mass of several million is present in most tissues as a component of the extracellular matrix. Elevated levels of serum HA have been reported in various diseases including liver diseases. Increases in serum HA correspond to the progression of liver diseases, including viral and non-viral diseases. Ding et al demonstrated that the elevated serum HA levels were closely related to the severity of liver fibrosis, particularly in LC[12]. In addition, Procollagen III peptide, laminin, and type IV collagen with molecular masses of 45000, 400000 and 67000, respectively, are also extracellular matrix glycoproteins and have been reported to be correlated to necrosis and inflammation as well as fibrosis in patients with chronic hepatitis and LC[13]. However, the diagnostic value, i.e. sensitivity and specificity, of these markers for patients with cirrhosis has not been satisfactory so far. Use of multiple markers led to 90% sensitivity at most in diagnosing cirrhosis, but variable specificity was about 60%[14].
SELDI-TOF mass spectrometry is a recently described affinity-based mass spectrometric method that combines chromatography and mass spectrometry. This novel technology has been used for protein or peptide biomarker identification, biomolecular interactions and post-translational modifications. Protein chip technology has proven to be useful in the discovery of potential diagnostic markers for prostate[15-17], bladder[18], ovarian[19], breast[20-22], lung cancers[23], and pancreatic ductal adenocarcinoma. However, using it to discover new biomarkers of HBV induced diseases has not been addressed before. To identify potential biomarkers that can detect HBV induced LC, protein profiles of serum samples from LC patients were compared with those from the non-cirrhotic controls. Biomarker Pattern’s Software was used to identify two peaks differentially presented in control healthy and HCC serum samples compared with LC samples. The top-scored two peaks with Mr 7772 and 3933 were finally selected. These two proteins generated a sensitivity of 96% and specificity of 77.8%. It is difficult to find a good single marker associated with diseases because of the differences among patients’ age, gender, diet and genes. Furthermore, double-blind test was used to determine the sensitivity and specificity of the model. A sensitivity of 80%, specificity of 81.8% and positive predictive value of 75% for the study population were obtained when comparing LC versus non-cirrhotic (HCC/healthy men) groups. The low-molecular-mass serum proteins are apparently different from known non-invasive serum biomarkers for LC in many aspects and might be acceptable for diagnosis and assessment of HBV associated LC.
| 2. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 537] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 155] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Issaq HJ, Veenstra TD, Conrads TP, Felschow D. The SELDI-TOF MS approach to proteomics: protein profiling and biomarker identification. Biochem Biophys Res Commun. 2002;292:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 374] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | He QY, Lau GK, Zhou Y, Yuen ST, Lin MC, Kung HF, Chiu JF. Serum biomarkers of hepatitis B virus infected liver inflammation: a proteomic study. Proteomics. 2003;3:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 401] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 7. | Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8-24. [PubMed] |
| 8. | Xiang Y, Qian L, Wang B. [Reversion of HBV-related liver fibrosis and early cirrhosis by baicao rougan capsule]. Zhongguo Zhongxiyi Jiehe Zazhi. 1999;19:709-711. [PubMed] |
| 9. | Kozłowska J, Łoch T, Jabłońska J, Cianciara J. [Biochemical markers of fibrosis in chronic hepatitis and liver cirrhosis of viral origin]. Przegl Epidemiol. 2001;55:451-458. [PubMed] |
| 10. | Chang TT, Lin HC, Lee SD, Tsai YT, Lee FY, Jeng FS, Wu JC, Yeh PS, Lo KJ. Clinical significance of serum type-III procollagen aminopropeptide in hepatitis B virus-related liver diseases. Scand J Gastroenterol. 1989;24:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Luo R, Yang S, Xie J, Zhao Z, He Y, Yao J. [Diagnostic value of five serum markers for liver fibrosis]. Zhonghua Ganzangbing Zazhi. 2001;9:148-150. [PubMed] |
| 12. | Ding H, Chen Y, Feng X, Liu D, Wu A, Zhang L. [Correlation between liver fibrosis stage and serum liver fibrosis markers in patients with chronic hepatitis B]. Zhonghua Ganzangbing Zazhi. 2001;9:78-80. [PubMed] |
| 13. | Castera L, Hartmann DJ, Chapel F, Guettier C, Mall F, Lons T, Richardet JP, Grimbert S, Morassi O, Beaugrand M. Serum laminin and type IV collagen are accurate markers of histologically severe alcoholic hepatitis in patients with cirrhosis. J Hepatol. 2000;32:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Oh S, Afdhal NH. Hepatic fibrosis: are any of the serum markers useful? Curr Gastroenterol Rep. 2001;3:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Qu Y, Adam BL, Yasui Y, Ward MD, Cazares LH, Schellhammer PF, Feng Z, Semmes OJ, Wright GL. Boosted decision tree analysis of surface-enhanced laser desorption/ionization mass spectral serum profiles discriminates prostate cancer from noncancer patients. Clin Chem. 2002;48:1835-1843. [PubMed] |
| 16. | Adam BL, Qu Y, Davis JW, Ward MD, Clements MA, Cazares LH, Semmes OJ, Schellhammer PF, Yasui Y, Feng Z. Serum protein fingerprinting coupled with a pattern-matching algorithm distinguishes prostate cancer from benign prostate hyperplasia and healthy men. Cancer Res. 2002;62:3609-3614. [PubMed] |
| 17. | Bañez LL, Prasanna P, Sun L, Ali A, Zou Z, Adam BL, McLeod DG, Moul JW, Srivastava S. Diagnostic potential of serum proteomic patterns in prostate cancer. J Urol. 2003;170:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Adam BL, Vlahou A, Semmes OJ, Wright GL. Proteomic approaches to biomarker discovery in prostate and bladder cancers. Proteomics. 2001;1:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2358] [Cited by in RCA: 1925] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 20. | Li J, Zhang Z, Rosenzweig J, Wang YY, Chan DW. Proteomics and bioinformatics approaches for identification of serum biomarkers to detect breast cancer. Clin Chem. 2002;48:1296-1304. [PubMed] |
| 21. | Paweletz CP, Trock B, Pennanen M, Tsangaris T, Magnant C, Liotta LA, Petricoin EF. Proteomic patterns of nipple aspirate fluids obtained by SELDI-TOF: potential for new biomarkers to aid in the diagnosis of breast cancer. Dis Markers. 2001;17:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Coombes KR, Fritsche HA, Clarke C, Chen JN, Baggerly KA, Morris JS, Xiao LC, Hung MC, Kuerer HM. Quality control and peak finding for proteomics data collected from nipple aspirate fluid by surface-enhanced laser desorption and ionization. Clin Chem. 2003;49:1615-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Xiao XY, Tang Y, Wei XP, He DC. A preliminary analysis of non-small cell lung cancer biomarkers in serum. Biomed Environ Sci. 2003;16:140-148. [PubMed] |
Co-first-authors: Xiao-Dong Zhu and Wei-Hua Zhang
Edited by Chen WW Proofread by Zhu LH and Xu FM