Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1686
Revised: February 4, 2004
Accepted: February 11, 2004
Published online: June 1, 2004
We report a case of fatal liver failure due to reactivation of lamivudine-resistant HBV. A 53-year-old man was followed since 1998 for HBV-related chronic hepatitis. Serum HBV-DNA was 150 MEq/mL (branched DNA signal amplification assay) and ALT levels fluctuated between 50-200 IU/L with no clinical signs of liver cirrhosis. Lamivudine (100 mg/d) was started in May 2001 and serum HBV-DNA subsequently decreased below undetectable levels. In May 2002, serum HBV-DNA had increased to 410 MEq/mL, along with ALT flare (226 IU/L). The YMDD motif in the DNA polymerase gene had been replaced by YIDD. Lamivudine was continued and ALT spontaneously decreased to the former levels. On Oct 3 the patient presenting with general fatigue, nausea and jaundice was admitted to our hospital. The laboratory data revealed HBV reactivation and liver failure (ALT: 1828 IU/L, total bilirubin: 10 mg/dL, and prothrombin INR: 3.24). For religious reasons, the patient and his family refused blood transfusion, plasma exchange and liver transplantation. The patient died 10 d after admission. The autopsy revealed remarkable liver atrophy.
- Citation: Kagawa T, Watanabe N, Kanouda H, Takayama I, Shiba T, Kanai T, Kawazoe K, Takashimizu S, Kumaki N, Shimamura K, Matsuzaki S, Mine T. Fatal liver failure due to reactivation of lamivudine-resistant HBV mutant. World J Gastroenterol 2004; 10(11): 1686-1687
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1686.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1686
The emergence of lamivudine-resistant hepatitis B virus (HBV) mutant is relatively frequent after long-term lamivudine treatment. Liaw et al[1] reported acute exacerbation in 41% of lamivudine-treated patients who developed YMDD mutation, recovering mostly with HBeAg seroconversion. Conversely, evolution toward acute liver failure is rare but possibly fatal as published in 2001 by Kim et al[2]. We report here a new case of fatal liver failure consecutive to the emergence of a lamivudine-resistant mutant HBV.
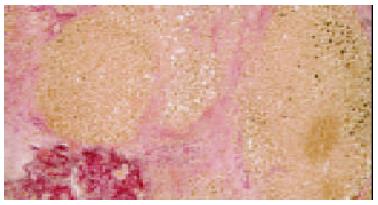
This 53-year-old man had been followed since 1998 for HBV-related chronic hepatitis. He was positive for HBs and HBe antigens. Serum HBV-DNA was 150 MEq/mL (branched DNA signal amplification assay)[3]. Alanine aminotransferase (ALT) fluctuated between 50-200 IU/L with no clinical signs of liver cirrhosis. Serum albumin and prothrombin time were normal. Lamivudine (100 mg/d) was started in May 2001 (Figure 1). Subsequently, serum HBV-DNA decreased below undetectable level (October 2001). In May 2002, serum HBV-DNA had increased to 410 MEq/mL, along with ALT flare (226 IU/L). Mini-sequencing[4] showed that the YMDD motif in the DNA polymerase gene had been replaced by YIDD, supporting reactivation was due to the emergence of a lamivudine-resistant HBV mutant. Lamivudine was continued and ALT spontaneously decreased to the former levels. To improve a pre-existing non-insulin dependent diabetes mellitus (serum HgbA1c: 6.5%-7.6%) acarbose (300 mg/d) was initiated on Aug 20, 2002, but discontinued after only 3 d due to flatulence. At the next outpatient clinic visit (Sept 17), the patient presented no clinical sign of liver disorder, so acarbose was restarted. In fact, laboratory investigations performed prior to restarting acarbose revealed that HBV-DNA had dramatically increased to 3800 MEq/mL while ALT was 9 times that of the upper limit of normal (357 IU/L). On Oct 3, the patient presenting with general fatigue, nausea and jaundice was admitted to the hospital. ALT was 1828 IU/L, total and direct bilirubin, 10 and 5.8 mg/dL, respectively, and prothrombin INR 3.24. Antinuclear and antismooth muscle antibodies as well as HIV tests were negative. Despite discontinuation of acarbose, liver failure developed and hepatic encephalopathy appeared. For religious reasons, the patient and his family refused blood transfusion, plasma exchange and liver transplantation. The patient died 10 d after admission. The autopsy revealed liver atrophy (620 g) and panlobular necrosis surrounded by extensive fibrosis consisting of immature collagen (Figure 2). Lymphoid cell infiltration was observed in the fibrosis.
The reactivation of HBV prior to any clinical sign and administration of acarbose supports that the liver failure reported here is consecutive to the rapid proliferation of lamivudine-resistant HBV (YIDD). Although rare, acute liver failure associated with lamivudine-resistant HBV is reported in the literature, including one successfully treated with liver transplant[5], one co-infected with HIV[6], two with advanced cirrhosis[7] and one immunocompetent patient[2]. The last case was a Korean male, who developed fatal liver failure after 20 mo of lamivudine therapy. The background is similar to our patient, indicating that Asian males might be susceptible to this type of mutants. Lamivudine-resistant HBV is sensitive to adefovir dipivoxil[8], a nucleotide analogue, shown to be effective for chronic HBV infection[9]. Adefovir dipivoxil, unavailable in Japan, could not be used in our case.
Acarbose is a pseudotetrasaccaride acting by competitive inhibition of intestinal alpha-glucosidases, indicated for the treatment of type II diabetes mellitus. The incidence of acarbose-related liver injury is low although a few severe cases were reported[10,11]. It is unclear whether acarbose might have aggravated the liver injury consecutive to the mutation-related relapse of viral activity. In any case, as stressed in the Summary of Product Characteristics for acarbose “Glucobay is contra-indicated in patients with hepatic impairment”. The above medical history supports closely monitoring patients with lamivudine-resistant HBV even in immunocompetent patients and, once reactivation occurs, adefovir dipivoxil should be administered.
We thank Dr. Jean C. Delumeau for his help with preparation of manuscript.
| 1. | Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 403] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 2. | Kim JW, Lee HS, Woo GH, Yoon JH, Jang JJ, Chi JG, Kim CY. Fatal submassive hepatic necrosis associated with tyrosine-methionine-aspartate-aspartate-motif mutation of hepatitis B virus after long-term lamivudine therapy. Clin Infect Dis. 2001;33:403-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Hendricks DA, Stowe BJ, Hoo BS, Kolberg J, Irvine BD, Neuwald PD, Urdea MS, Perrillo RP. Quantitation of HBV DNA in human serum using a branched DNA (bDNA) signal amplification assay. Am J Clin Pathol. 1995;104:537-546. [PubMed] |
| 4. | Kobayashi S, Shimada K, Suzuki H, Tanikawa K, Sata M. Development of a new method for detecting a mutation in the gene encoding hepatitis B virus reverse transcriptase active site (YMDD motif). Hepatol Res. 2000;17:31-42. [DOI] [Full Text] |
| 5. | de Man RA, Bartholomeusz AI, Niesters HG, Zondervan PE, Locarnini SA. The sequential occurrence of viral mutations in a liver transplant recipient re-infected with hepatitis B: hepatitis B immune globulin escape, famciclovir non-response, followed by lamivudine resistance resulting in graft loss. J Hepatol. 1998;29:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bonacini M, Kurz A, Locarnini S, Ayres A, Gibbs C. Fulminant hepatitis B due to a lamivudine-resistant mutant of HBV in a patient coinfected with HIV. Gastroenterology. 2002;122:244-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Malik AH, Lee WM. Hepatitis B therapy: the plot thickens. Hepatology. 1999;30:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [PubMed] |
| 10. | Fujimoto Y, Ohhira M, Miyokawa N, Kitamori S, Kohgo Y. Acarbose-induced hepatic injury. Lancet. 1998;351:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Carrascosa M, Pascual F, Aresti S. Acarbose-induced acute severe hepatotoxicity. Lancet. 1997;349:698-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Edited by Ma JY and Xu FM