©The Author(s) 2026.
World J Gastroenterol. Feb 28, 2026; 32(8): 113299
Published online Feb 28, 2026. doi: 10.3748/wjg.v32.i8.113299
Published online Feb 28, 2026. doi: 10.3748/wjg.v32.i8.113299
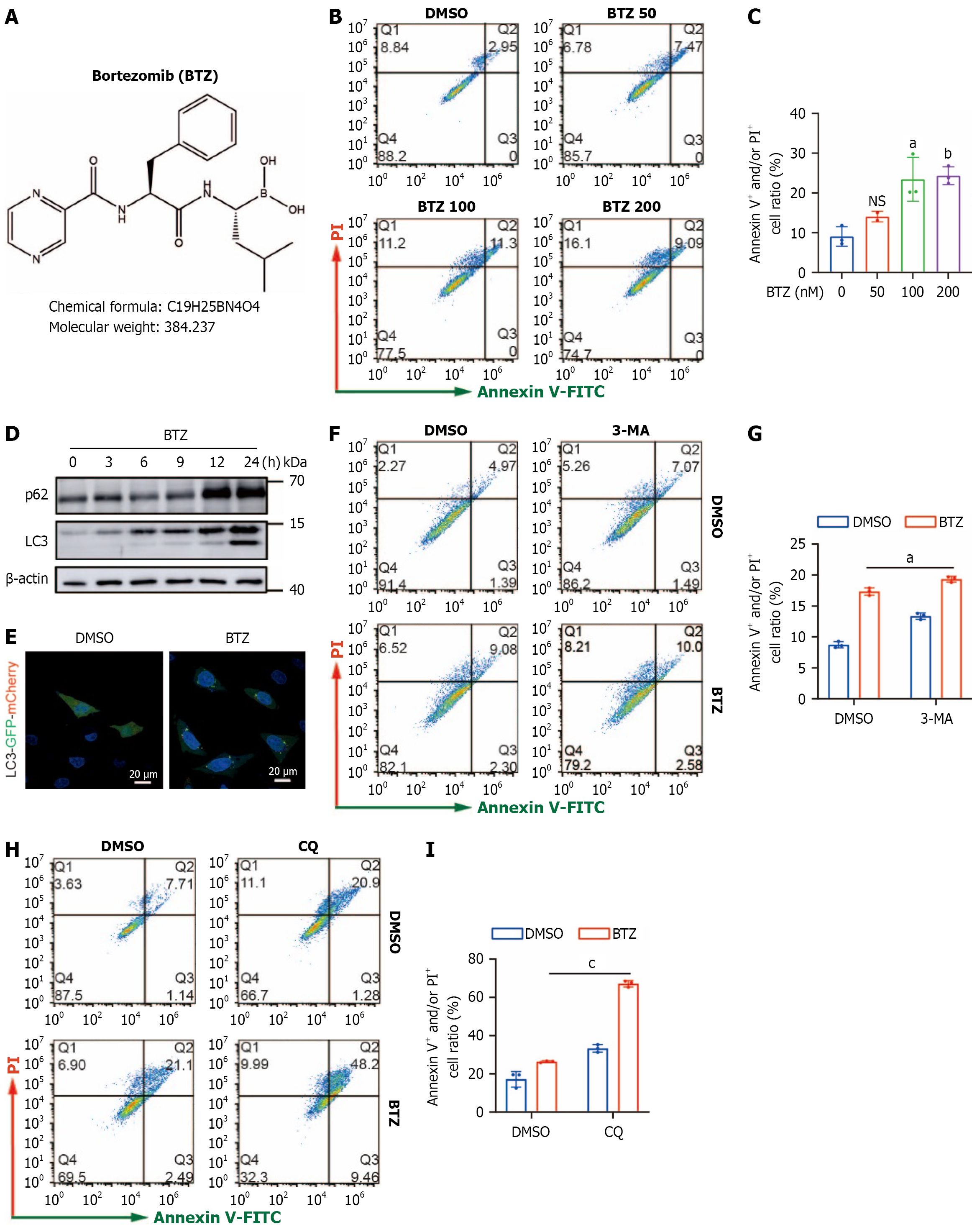
Figure 1 Effect of bortezomib on apoptosis and autophagy in MGC-803 gastric cancer cells.
A: Structure of bortezomib (BTZ); B and C: MGC-803 cells were treated with different concentration of BTZ for 24 hours, followed by flow cytometry for cell death. Representative images (B) and quantification data (C) are shown; D: MGC-803 cells were treated with BTZ (100 nM). The cell lysates were subjected to western blotting using antibody against p62 and light chain 3 (LC3), and β-actin as loading control; E: MGC-803 cells were transfected using lentivirus containing mCherry- green fluorescent protein-LC3, and treated with BTZ (100 nM) for 24 hours. Immunofluorescence analysis was performed for evaluating autophagosome (scale bar: 20 μm); F-I: MGC-803 cells were treated with BTZ (100 nM) with or without 3-methyladenine (5 mmol/L), and chloroquine (40 μM) for 24 hours, followed by cell death detection. aP < 0.05. bP < 0.01. cP < 0.001. BTZ: Bortezomib; DMSO: Dimethyl sulfoxide; V-FITC: Annexin V-fluorescein isothiocyanate; PI: Propidium iodide; LC3: Light chain 3; NS: No significance; GFP: Green fluorescent protein; 3-MA: 3-methyladenine; CQ: Chloroquine.
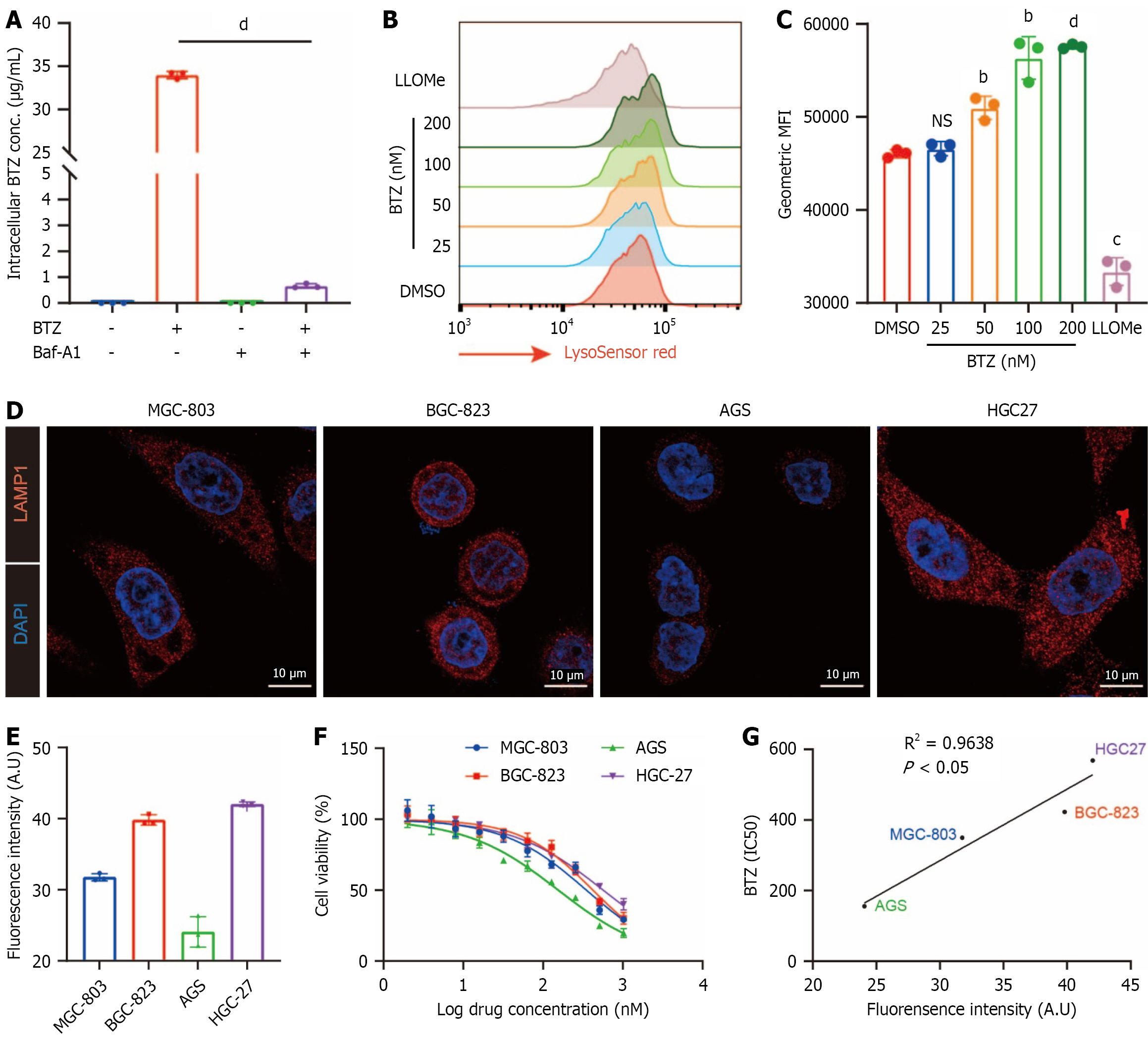
Figure 2 Lysosome affects the anticancer effect of bortezomib.
A: Intracellular drug concentration was examined using liquid chromatography/mass spectrometry after cells were treated with bortezomib (BTZ) (100 nM) and bafilomycin A1 (100 nM) alone or in combination for 6 hours; B and C: MGC-803 cells were treated with indicated concentration of BTZ and L-leucyl-L-leucine methyl ester hydrochloride (1 mmol/L) for 6 hours, followed by staining of LysoSensor Green DND-189. Representative images (B) and quantification data (C) of flow cytometry were shown; D and E: Representative images (D) and quantified results (E) of immunofluorescent analysis for LAMP1 expression in different gastric cancer cell lines (scale bar: 10 μm); F: Cell viability assay was performed in different gastric cancer cells treated with BTZ; G: Spearman correlation was determined between half-maximal inhibitory concentration value for BTZ and cellular LAMP1 expression. bP < 0.01. cP < 0.001. dP < 0.0001. BTZ: Bortezomib; Baf-A1: Bafilomycin A1; LLOMe: L-leucyl-L-leucine methyl ester hydrochloride; DMSO: Dimethyl sulfoxide; MFI: Mean fluorescence intensity; DAPI: 4’,6-Diamidino-2-phenylindole; NS: No significance; IC50: Half-maximal inhibitory concentration.
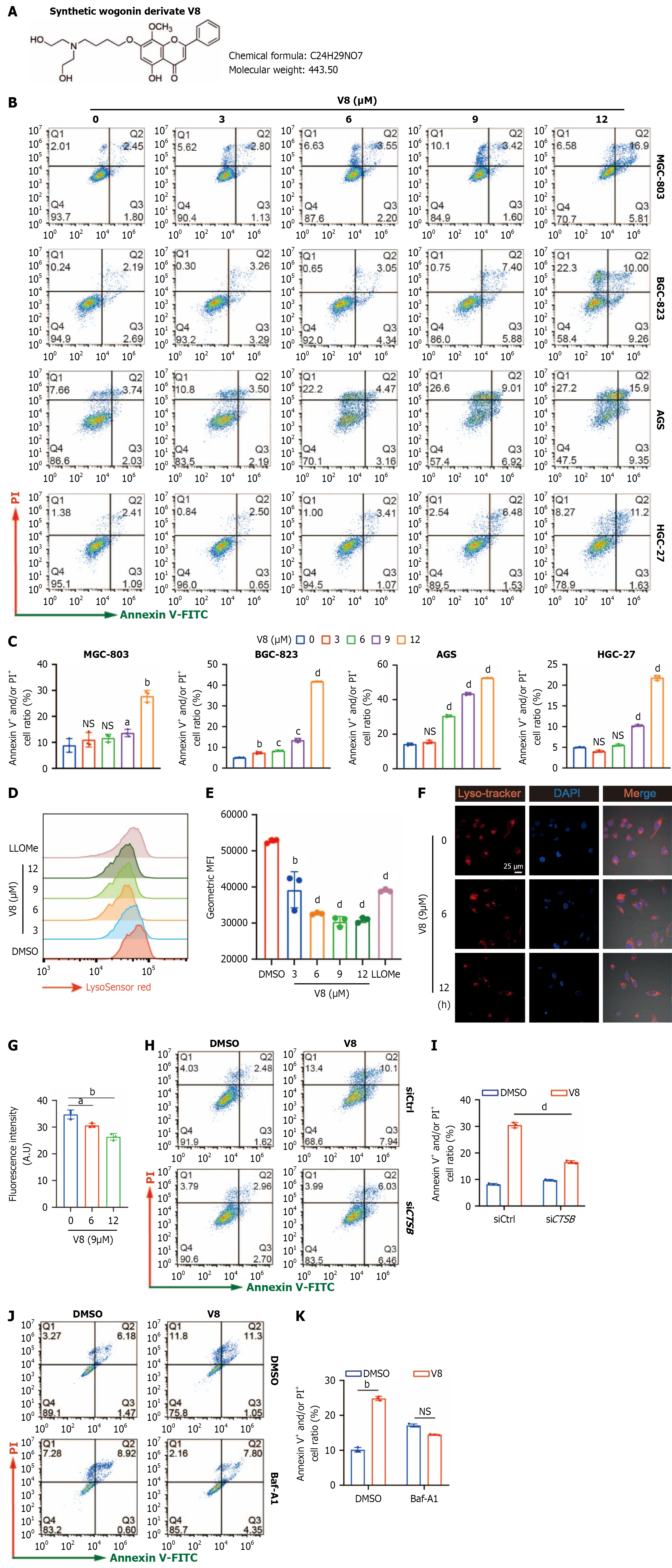
Figure 3 V8 induces lysosomal cell death in gastric cancer cells.
A: Structure of synthetic flavonoid derivate V8; B and C: Gastric cancer cells were treated with V8 at indicated concentration for 24 hours. Representative images (B) and quantified data (C) for cell death are shown; D and E: MGC-803 cells were treated with V8 and L-leucyl-L-leucine methyl ester hydrochloride (1 mmol/L) for 6 hours, followed by staining with LysoSensor Green DND-189 dye. Representative images (D) and quantification data (E) are shown; F and G: MGC-803 cells were treated with V8 (9 μM), followed by staining with LysoTracker red dye. Representative images and quantified intensity of immunofluorescence analysis are shown (scale bar: 25 μm); H-K: MGC-803 cells were transfected with small interfering RNA against CSTB gene or treated with bafilomycin A1 (100 nM), followed by V8 (12 μM) for 24 hours. Representative images (H and J) and quantification data (I and K) of flow cytometry analysis for cell death are shown. aP < 0.05. bP < 0.01. cP < 0.001. dP < 0.0001. V-FITC: Annexin V-fluorescein isothiocyanate; PI: Propidium iodide; LLOMe: L-leucyl-L-leucine methyl ester hydrochloride; DMSO: Dimethyl sulfoxide; MFI: Mean fluorescence intensity; DAPI: 4’,6-Diamidino-2-phenylindole; NS: No significance; Baf-A1: Bafilomycin A1; siCtrl: Negative control small interfering RNA; siCTSB: Small interfering RNA targeting CTSB.
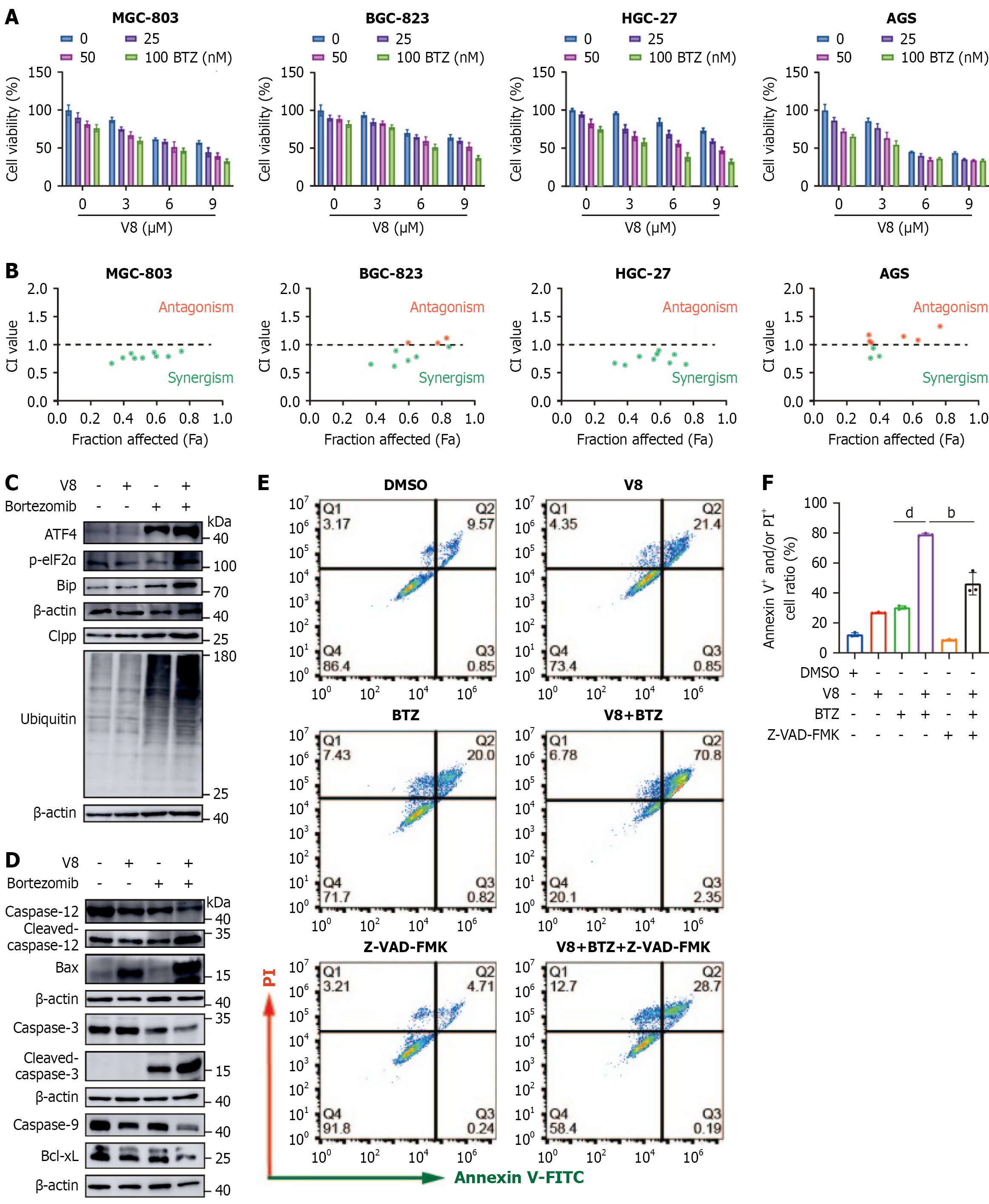
Figure 4 V8 synergizes with bortezomib to inhibit gastric cancer cell growth.
A: Cell viability assay was performed in different gastric cancer cells treated with V8 and bortezomib (BTZ) for 24 hours; B: Combination index was calculated in different gastric cancer cells; C and D: MGC-803 cells were treated with V8 (9 μM) and BTZ (100 nM) for 24 hours. Western blotting was carried out for analysis of indicated protein expression with β-actin as loading control; E and F: Cells were treated with V8 (9 μM), BTZ (100 nM), or benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethylketone (20 μM) for 24 hours, followed by flow cytometry assay for apoptosis analysis. Representative images (E) and quantitative data (F) are shown. bP < 0.01. dP < 0.0001. CI: Combination index; Bip: Immunoglobulin heavy chain binding protein; p-eIF2α: Phosphorylated eukaryotic translation initiation factor 2 alpha; Clpp: Caseinolytic mitochondrial matrix peptidase proteolytic subunit; DMSO: Dimethyl sulfoxide; BTZ: Bortezomib; V-FITC: Annexin V-fluorescein isothiocyanate; PI: Propidium iodide; Z-VAD-FMK: Benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethylketone.
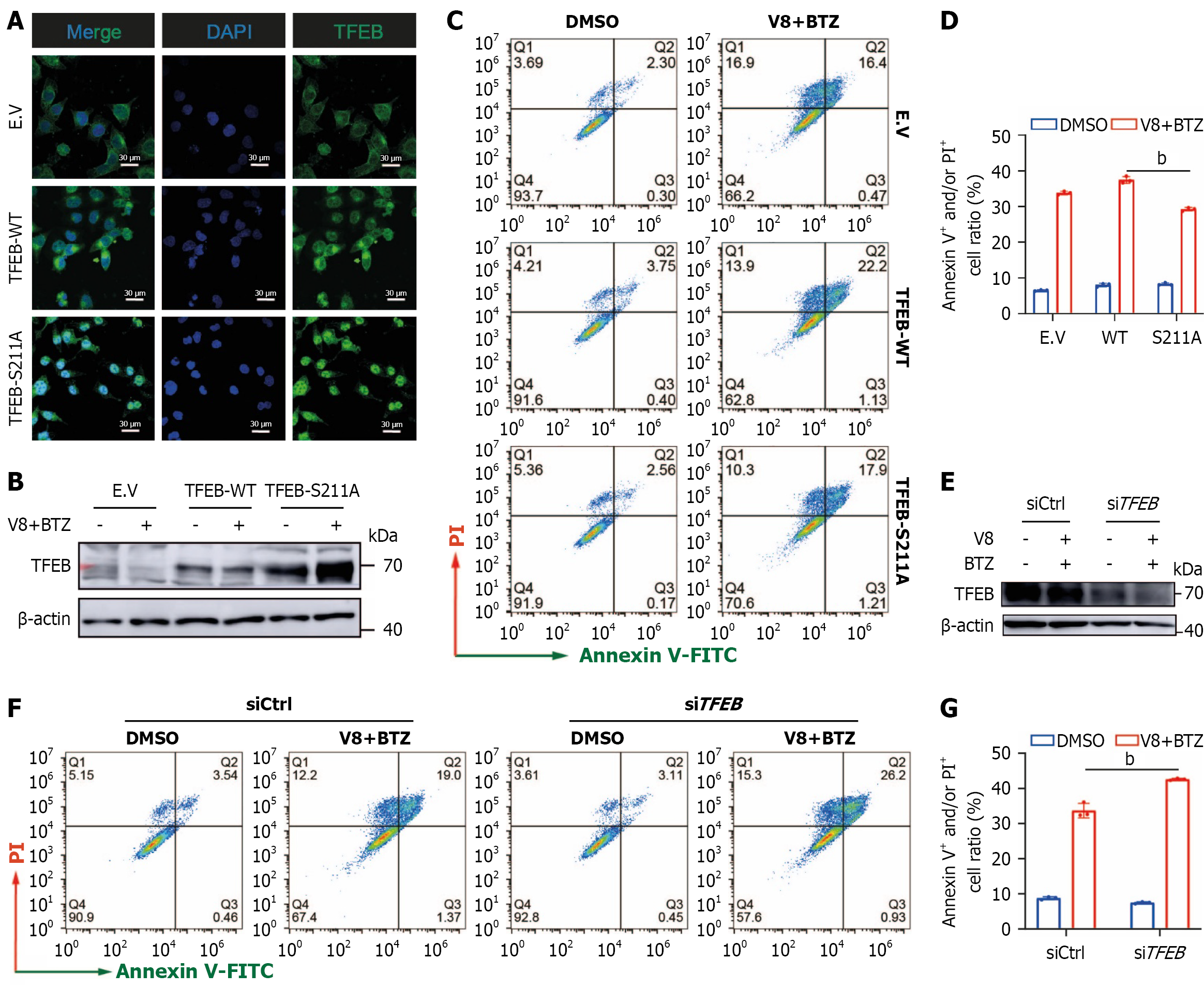
Figure 5 Role of transcription factor EB in cell apoptosis induced by V8 and bortezomib.
A: MGC-803 cells were transiently transfected with empty vector, wild-type, and S211A mutant form of transcription factor EB (TFEB) plasmids. Representative images of immunofluorescence assay are shown for analysis of cellular TFEB localization (scale bar: 30 μm); B-D: MGC-803 cells were transiently transfected with indicated plasmids (B), followed by treatment of V8 (9 μM) together with bortezomib (BTZ) (100 nM) for 24 hours. Representative images (C) and quantitative results (D) are shown; E: TFEB expression was examined by western blotting after transfection of specific small interfering RNA in MGC-803 cells; F and G: MGC-803 cells were treated by V8 (9 μM) combined with BTZ (100 nM) for 24 hours. Representative images (F) and quantified results (G) of cell apoptosis analysis are shown. bP < 0.01. DAPI: 4’,6-Diamidino-2-phenylindole; TFEB: Transcription factor EB; EV: Empty vector; WT: Wild-type; DMSO: Dimethyl sulfoxide; BTZ: Bortezomib; V-FITC: Annexin V-fluorescein isothiocyanate; PI: Propidium iodide; siCtrl: Negative control small interfering RNA; siTFEB: Small interfering RNA targeting TFEB.
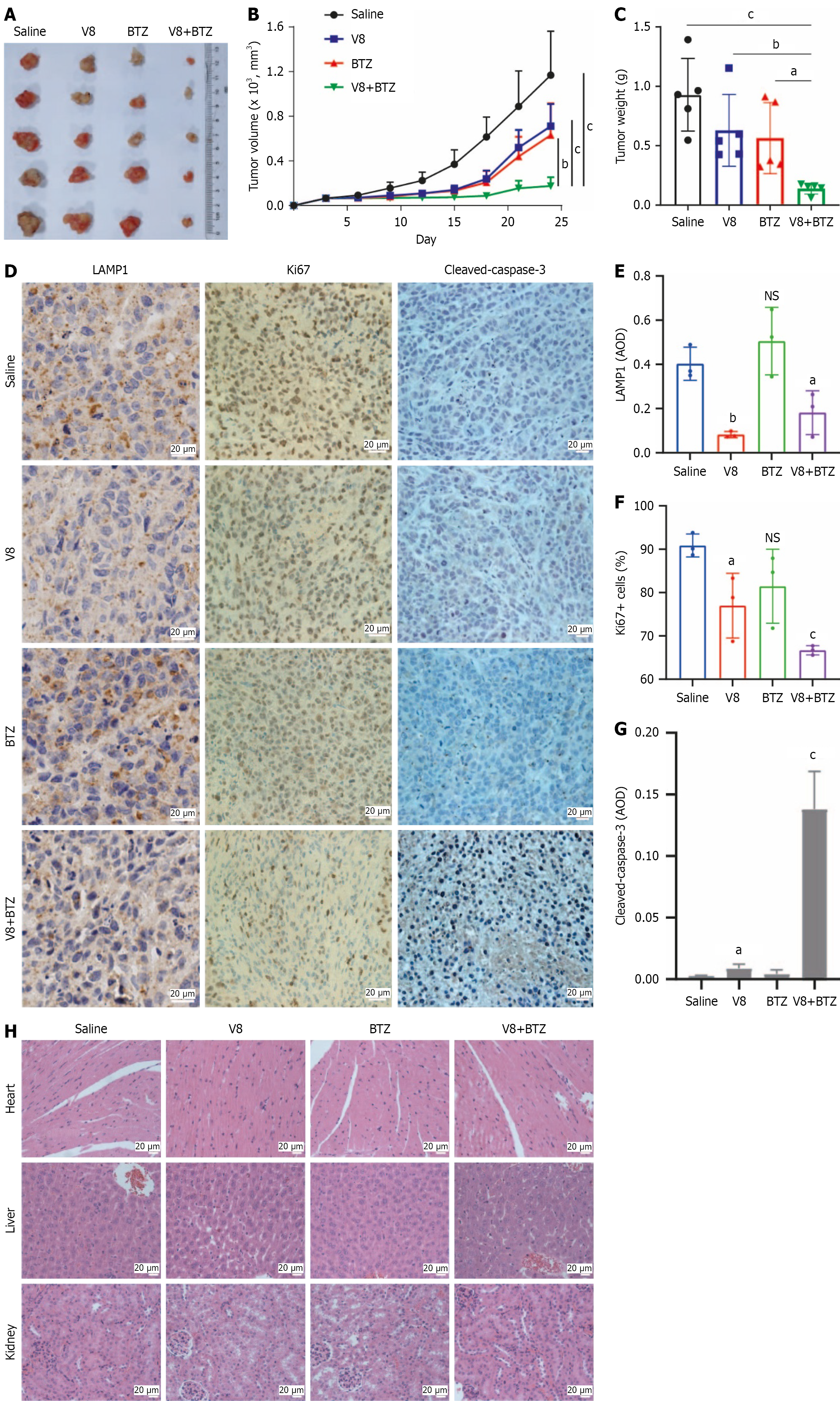
Figure 6 Effects of V8 and bortezomib on MGC-803 xenograft growth in vivo.
A: Images of tumor xenograft from different treatment groups; B: Tumor volume was monitored for different treatment groups during experimental period; C: Tumor weight was calculated for different groups at the end of experiment; D-G: Immunohistochemistry was performed for analysis of LAMP1, Ki67 and cleaved-caspase-3 expression. Representative images (D) and quantified data (E-G) are shown (scale bar: 20 μm); H: Hematoxylin and eosin staining was performed in tissue sections from heart, liver and kidney. Representative images are shown (scale bar: 20 μm). aP < 0.05. bP < 0.01. cP < 0.001. NS: No significance; BTZ: Bortezomib; AOD: Average optical density.
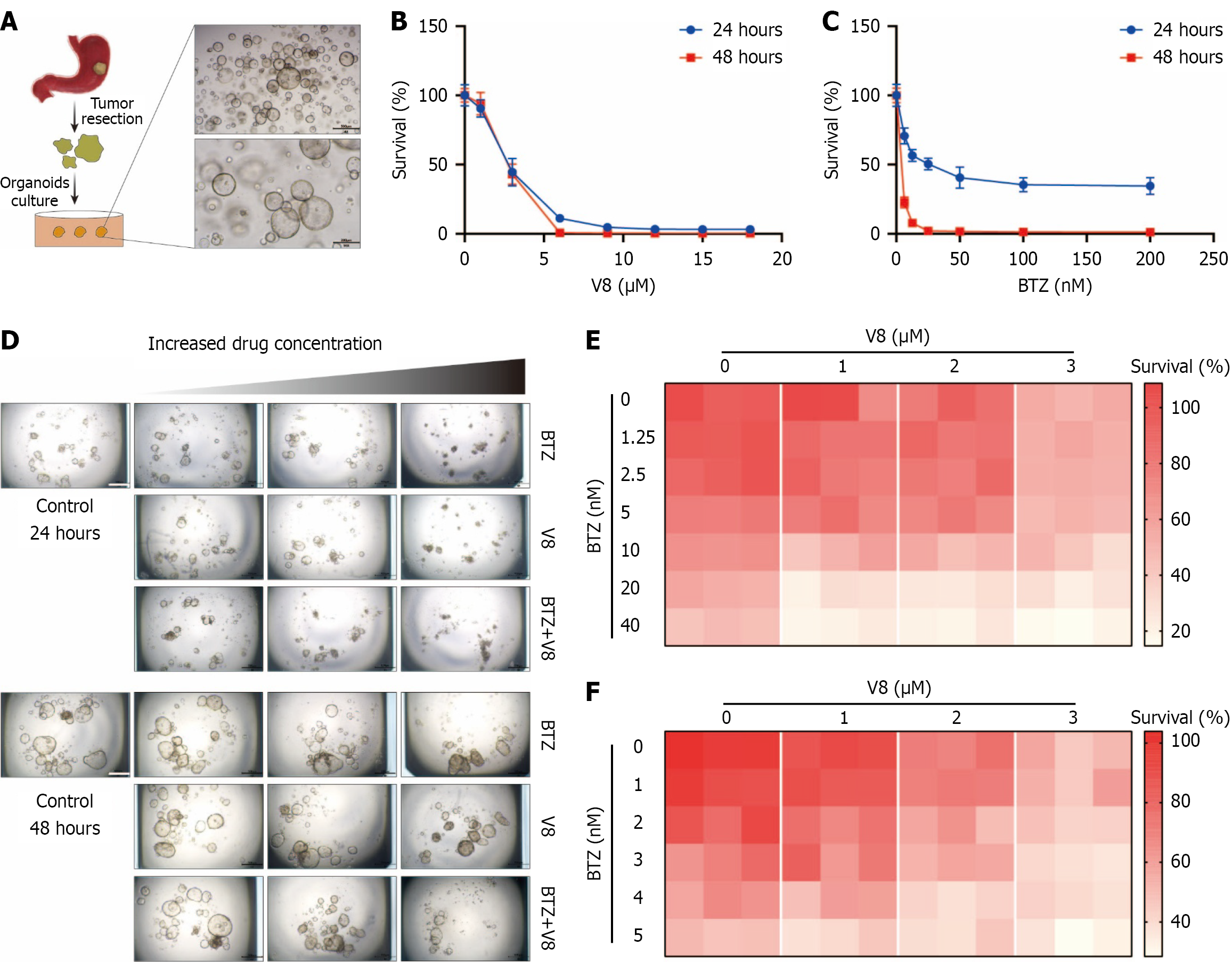
Figure 7 Combination of V8 and bortezomib suppresses patient-derived organoid growth.
A: Scheme of patient-derived organoid culture and representative images (scale bar: 500 μm); B and C: Sensitivity of organoids to V8 and bortezomib (BTZ) was examined for 24 hours and 48 hours; D-F: Viability of organoids was evaluated in response to combination of V8 and BTZ treatment for 24 hours and 48 hours. Representative images (D) and heatmap of cell viability (E: 24 hours; F: 48 hours) are shown (scale bar: 500 μm). BTZ: Bortezomib.
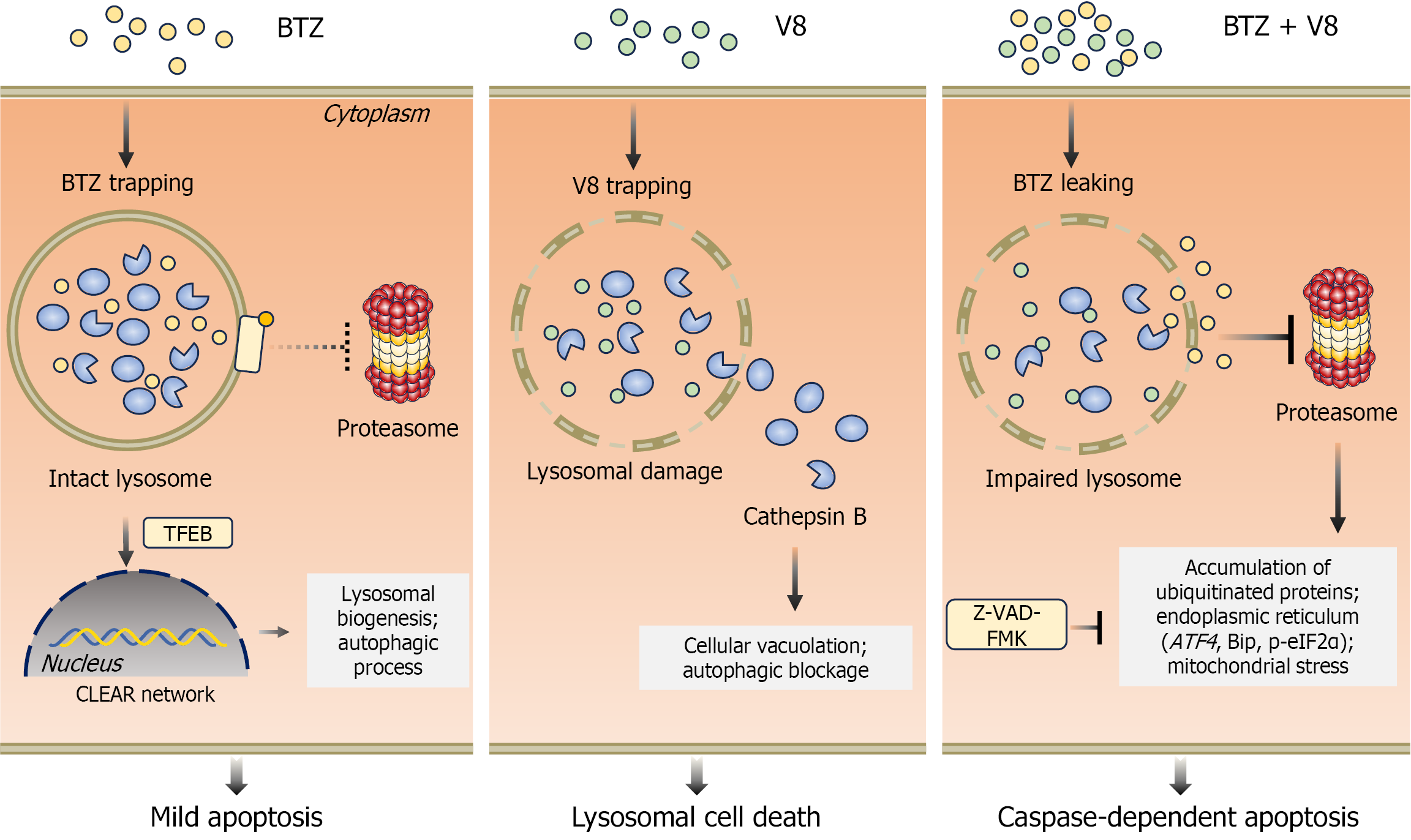
Figure 8 Schematic diagram illustrating the mechanism by which V8 synergizes with bortezomib to induce cell death in gastric cancer cells.
Bortezomib (BTZ) alone is sequestered in lysosomes, resulting in insufficient proteasome inhibition and mild apoptosis. V8 alone induces lysosomal damage and cell death via lysosomal membrane permeabilization and CTSB release. V8 disrupts lysosomes to release sequestered BTZ, enabling potent proteasome inhibition that triggers proteotoxic stress (ubiquitinated protein accumulation, endoplasmic reticulum stress, and mitochondrial stress), ultimately culminating in caspase-dependent apoptosis. BTZ: Bortezomib; TFEB: Transcription factor EB; CLEAR: Coordinated lysosomal expression and regulation; Bip: Immunoglobulin heavy chain binding protein; p-eIF2α: Phosphorylated eukaryotic translation initiation factor 2 alpha; Z-VAD-FMK: Benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethylketone.
- Citation: Li SC, Shao SZ, Zhang YH, Zhou Y, Shang WT, Gao Y, He QB, Guo QL, Guo CY, Zhang XB. Wogonin derivative V8 enhances bortezomib efficacy in gastric carcinoma by disrupting lysosome-mediated drug resistance. World J Gastroenterol 2026; 32(8): 113299
- URL: https://www.wjgnet.com/1007-9327/full/v32/i8/113299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i8.113299