©The Author(s) 2026.
World J Gastroenterol. Feb 14, 2026; 32(6): 113804
Published online Feb 14, 2026. doi: 10.3748/wjg.v32.i6.113804
Published online Feb 14, 2026. doi: 10.3748/wjg.v32.i6.113804
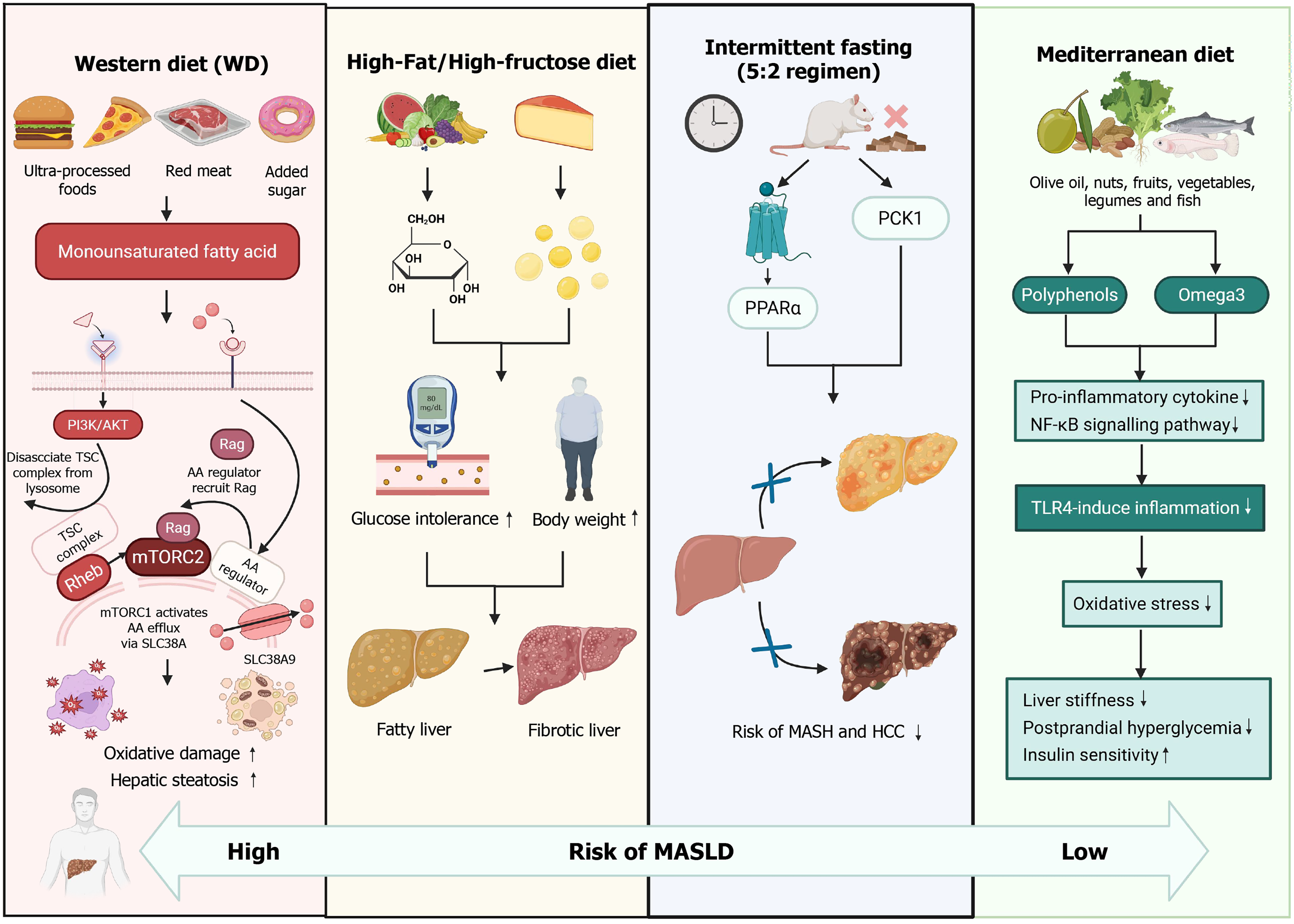
Figure 1 Comparative effects of dietary patterns on hepatic metabolism, inflammation, and metabolic dysfunction-associated steatotic liver disease risk.
The Western diet, characterized by high intake of ultra-processed foods, red meat, and added sugars, exacerbates hepatic steatosis, glucose intolerance, and bodyweight gain. This dietary pattern promotes proinflammatory cytokine release, nuclear factor kappa B signaling, and toll-like receptor 4-mediated inflammation, leading to oxidative stress, liver stiffness, and fibrosis. The high-fat/high-fructose diet rapidly increases blood glucose and bodyweight, inducing metabolic dysfunction and hepatic pathology. Consequently, the risk of metabolic dysfunction-associated steatohepatitis and hepatocellular carcinoma is markedly increased. In contrast, the Mediterranean diet, rich in monounsaturated fatty acids, omega-3 fatty acids, and polyphenols from nuts, fruits, vegetables, legumes, and fish, enhances insulin sensitivity and reduces hepatic lipid accumulation. Intermittent fasting (5:2 regimen) further augments these protective effects by upregulating phosphoenolpyruvate carboxykinase 1 and peroxisome proliferator-activated receptor alpha, thereby suppressing oxidative damage, inflammatory signaling, and postprandial hyperglycemia. PPARα: Peroxisome proliferator-activated receptor alpha; PCK1: Phosphoenolpyruvate carboxykinase 1; TSC: Tuberous sclerosis complex; AA: Amino acid; mTORC: Mechanistic target of rapamycin complex; MASH: Metabolic dysfunction-associated steatohepatitis; HCC: Hepatocellular carcinoma; NK-κB: Nuclear factor kappa B; TLR4: Toll-like receptor 4; MASLD: Metabolic dysfunction-associated steatotic liver disease; PI3K: Phosphatidylinositol 3-kinase; AKT: AKR mouse thymoma kinase; Rag: Ras-related GTP-binding protein; SLC38A: Solute carrier family 38 member A.
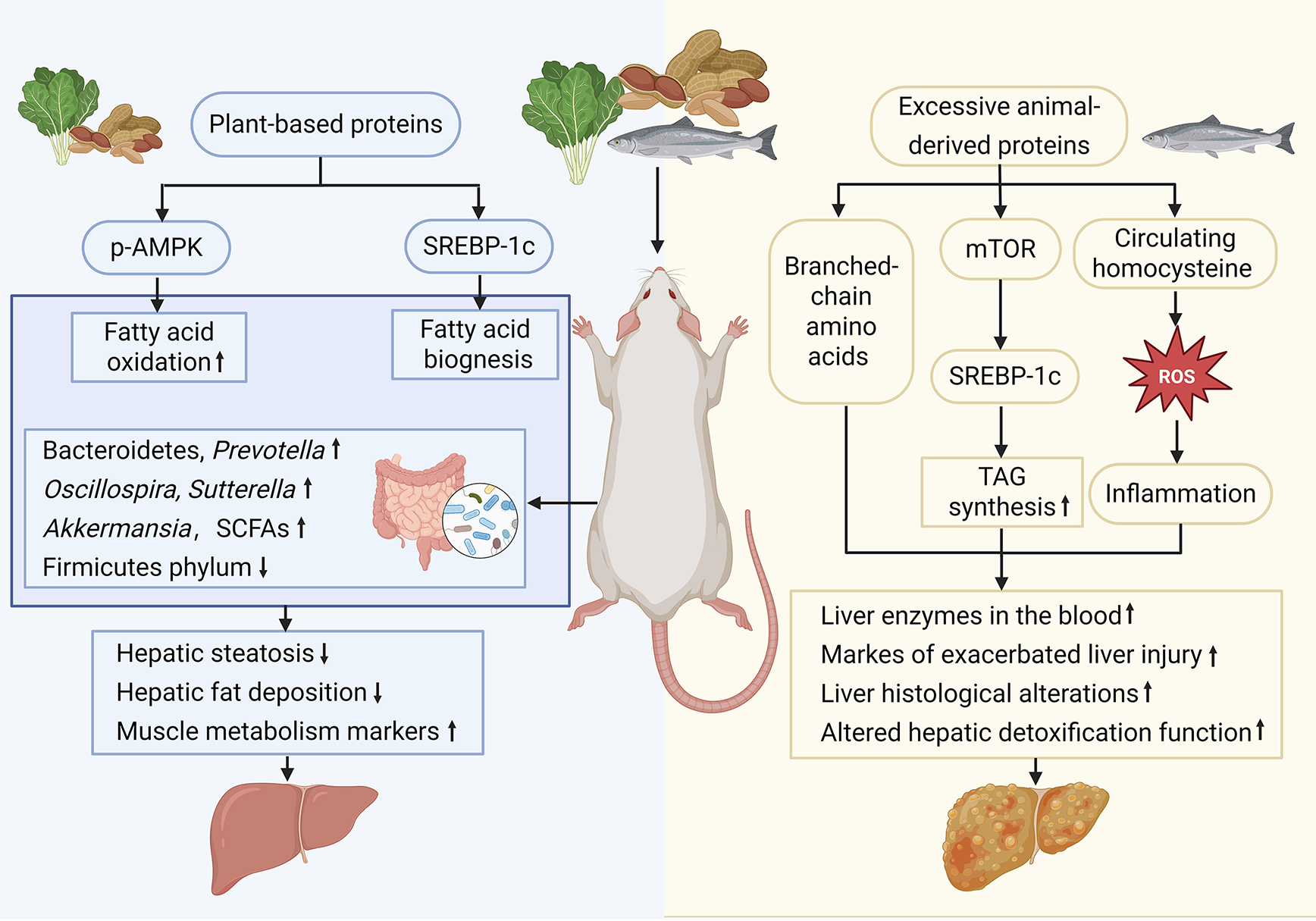
Figure 2 Animal experiments on the dual roles of high-protein diets in metabolic dysfunction-associated steatotic liver disease development and progression.
Compared with diets rich in animal-derived proteins, plant-based protein intake beneficially remodels hepatic lipid metabolism and gut microbiota. Some plant proteins are related to the increase of beneficial bacteria in the intestinal flora, but the intake of plant proteins is usually accompanied by fiber co-ingestion and resistant starch, conducive to the increase of beneficial bacteria. Diets high in animal protein upregulate mechanistic target of rapamycin and sterol regulatory element-binding protein-1c, elevate circulating branched-chain amino acids and homocysteine, and suppress phosphorylation-AMP-activated protein kinase, shifting the balance toward fatty acid biogenesis and away from oxidation, increasing triacylglycerol synthesis, hepatic steatosis, serum liver enzyme levels, and markers of liver injury. Conversely, plant-based proteins expand populations of Bacteroidetes, Prevotella, Akkermansia, and short-chain fatty acid producers while decreasing the relative abundance of Firmicutes, resulting in reduced hepatic fat deposition, ameliorated histological alterations, improved hepatic detoxification capacity, and attenuated systemic inflammation. p-AMPK: Phosphorylation-AMP-activated protein kinase; SREBP-1c: Sterol regulatory element-binding protein-1c; SCFAs: Short-chain fatty acids; mTOR: Mechanistic target of rapamycin; TAG: Triacylglycerol; ROS: Reactive oxygen species.
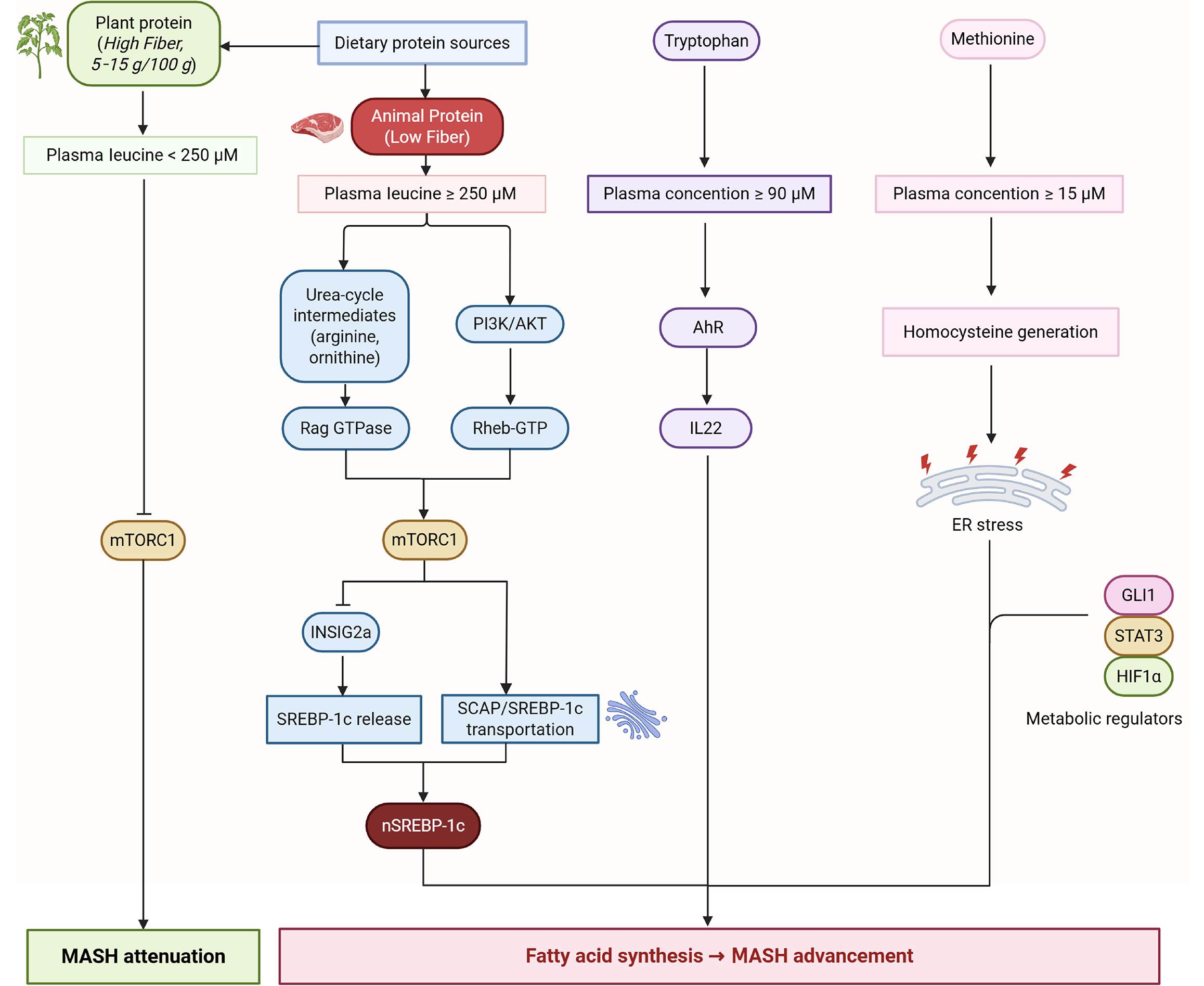
Figure 3 Dietary protein sources and metabolic regulation in metabolic dysfunction-associated steatotic liver disease progression.
This schematic illustrates the differential metabolic impacts of plant-derived vs animal-derived dietary proteins on systemic amino acid profiles and hepatic signaling pathways relevant to metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis. Plant proteins provide higher fiber and lower leucine bioavailability, maintaining plasma leucine < 250 μmol/L. In contrast, animal proteins (low fiber) elevate plasma leucine ≥ 250 μmol/L and increase methionine and tryptophan concentrations. Elevated leucine activates the Rag GTPase-Rheb-GTP axis, thereby stimulating mechanistic target of rapamycin complex 1 signaling and downstream transcriptional programs, including sterol regulatory element-binding protein-1c cleavage and nuclear translocation, promoting de novo lipogenesis. Concurrently, high methionine intake raises homocysteine levels, while arginine and ornithine serve as urea-cycle intermediates that modulate phosphatidylinositol 3-kinase/protein kinase B activity. These changes converge on endoplasmic reticulum stress, signal transducer and activator of transcription 3 and hypoxia inducible factor-1 alpha activation, and interleukin 22 induction, collectively exacerbating hepatic steatosis and inflammation. Conversely, plant-protein-associated lower leucine availability attenuates mechanistic target of rapamycin complex 1-sterol regulatory element-binding protein-1c signaling and favors metabolic dysfunction-associated steatohepatitis attenuation. mTORC1: Mechanistic target of rapamycin complex 1; PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; AhR: Aryl hydrocarbon receptor; IL22: Interleukin 22; SCAP: Sterol regulatory element-binding protein-cleavage-activating protein; SREBP-1c: Sterol regulatory element-binding protein-1c; ER: Endoplasmic reticulum; GLI1: Glioma-associated oncogene homolog 1; STAT3: Signal transducer and activator of transcription 3; HIF-1α: Hypoxia inducible factor-1 alpha; MASH: Metabolic dysfunction-associated steatohepatitis; Rag GTPase: Ras-related GTP-binding protein; Rheb: Ras homolog enriched in brain.
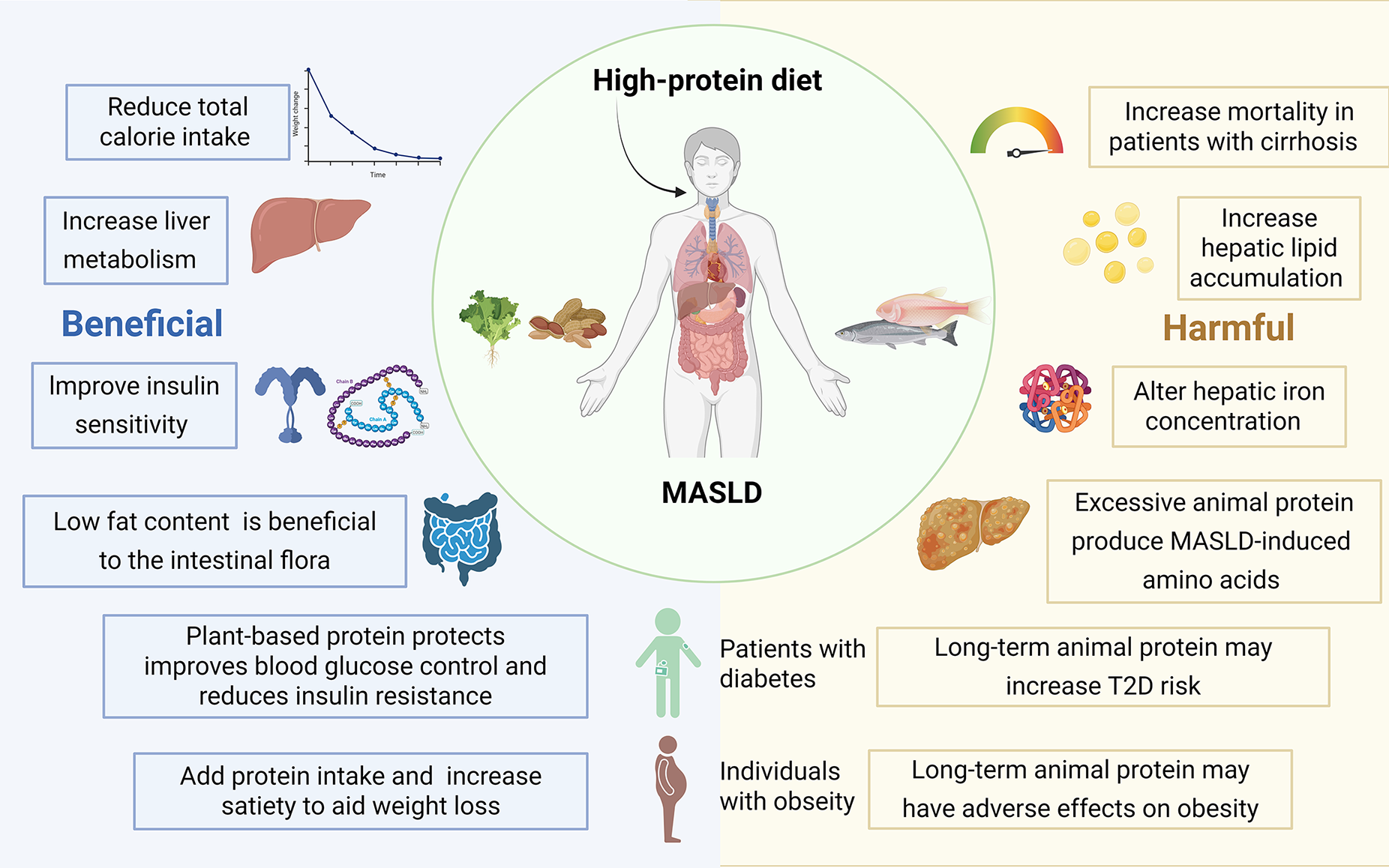
Figure 4 Clinical evidence of protein source and type on metabolic dysfunction-associated steatotic liver disease outcomes across study designs.
High-protein diets, especially those rich in animal-derived proteins, worsen metabolic dysfunction-associated steatotic liver disease by accelerating hepatic lipid accumulation, disturbing hepatic iron homeostasis, and generating metabolic dysfunction-associated steatotic liver disease-inducing amino acid metabolites that alter intestinal flora composition, heightening overall mortality in patients with cirrhosis. On the contrary, other studies have found a high-protein diet improves insulin sensitivity and rebalances gut microbial ecology, conferring a beneficial hepatic metabolic profile. MASLD: Metabolic dysfunction-associated steatotic liver disease; T2D: Type 2 diabetes.
- Citation: Yin HY, You QH, Zhang WJ, Ji G, Dang YQ. High-protein diets and metabolic dysfunction-associated steatotic liver disease: A double-edged sword in liver health. World J Gastroenterol 2026; 32(6): 113804
- URL: https://www.wjgnet.com/1007-9327/full/v32/i6/113804.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i6.113804