©The Author(s) 2026.
World J Gastroenterol. Feb 7, 2026; 32(5): 113505
Published online Feb 7, 2026. doi: 10.3748/wjg.v32.i5.113505
Published online Feb 7, 2026. doi: 10.3748/wjg.v32.i5.113505
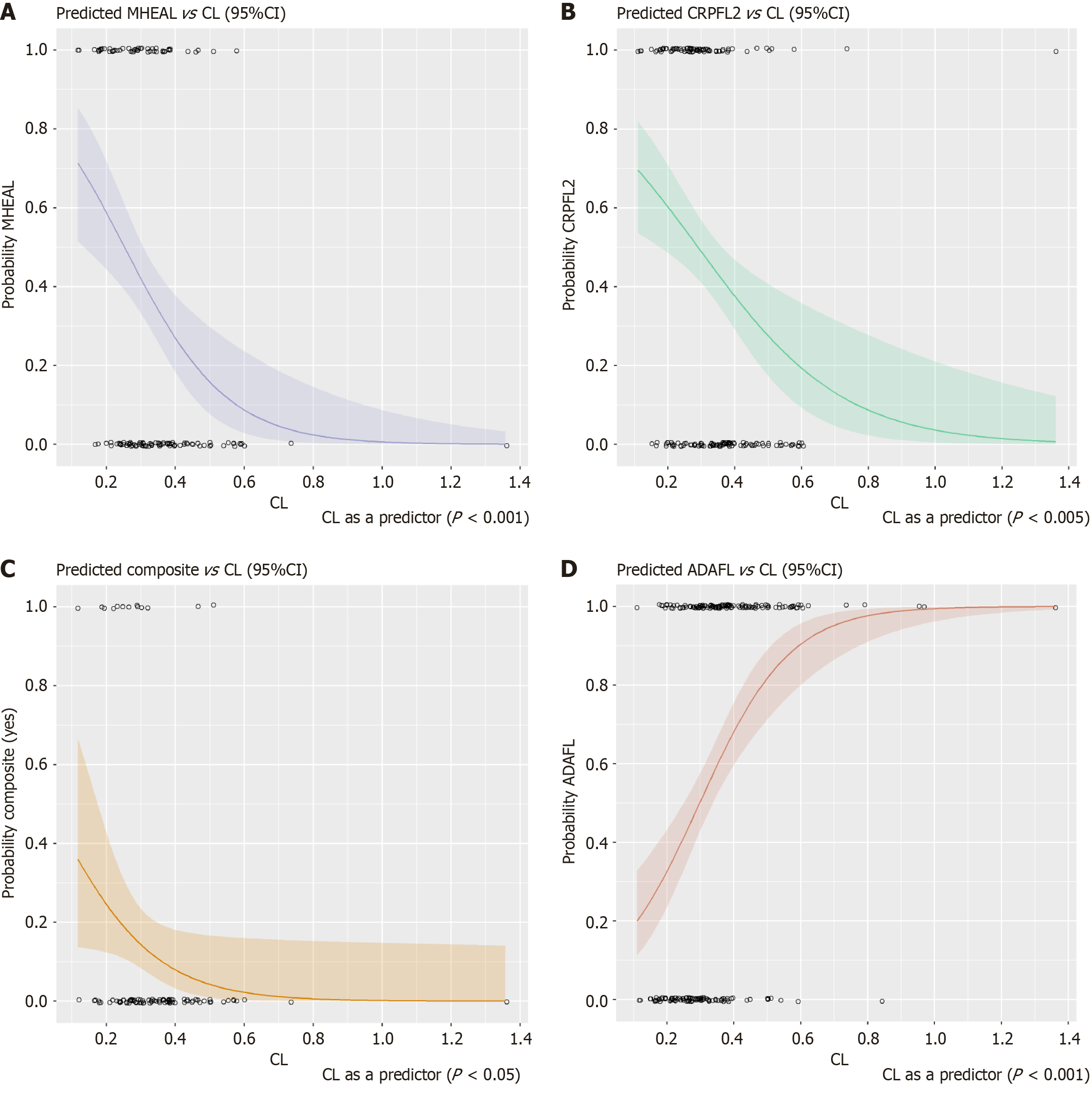
Figure 1 Probability outcomes predicted by initial clearance.
A: Mucosal healing; B: C-reactive protein normalization at 54 weeks; C: Composite endpoint; D: Development of antidrug antibody. Solid line is the median probability. Sharded areas represent the 95% confidence intervals (CIs), open symbols are individual clearance values. ADAFL: Positive for antidrug antibodies; CL: Clearance; Composite: A composite endpoint for mucosal healing, C-reactive protein normalization (less than 10 mg/L), Crohn’s Disease Activity Index, and fecal calprotectin at week 54; CRPFL2: C-reactive protein normalization (less than 10 mg/L) at week 54; MHEAL: Mucosal healing.
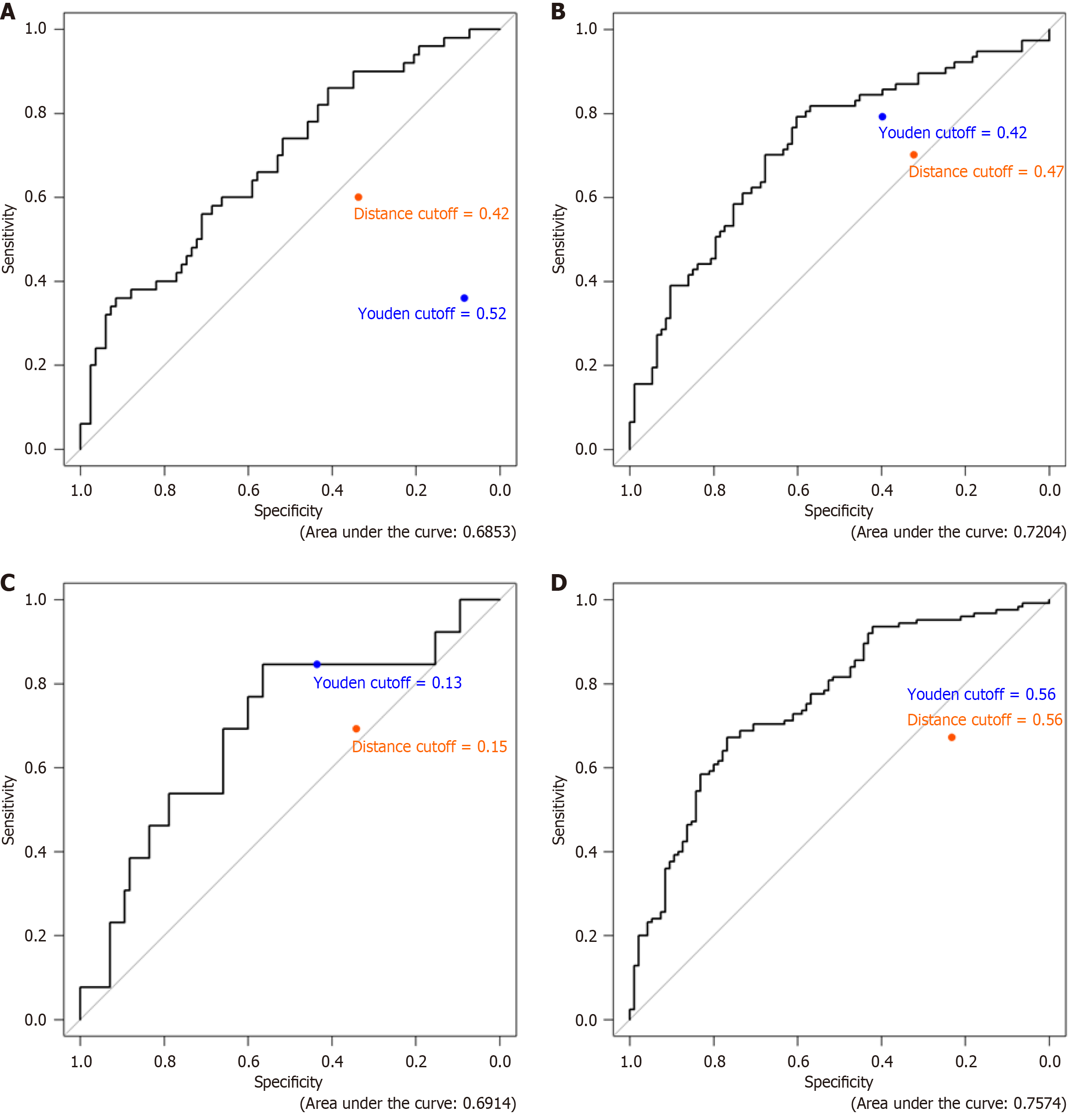
Figure 2 Receiver operating characteristic curves for mucosal healing, C-reactive protein normalization, combined endpoint and antidrug antibody status.
A: Mucosal healing; B: C-reactive protein; C: Composite endpoint; D: Antidrug antibody.
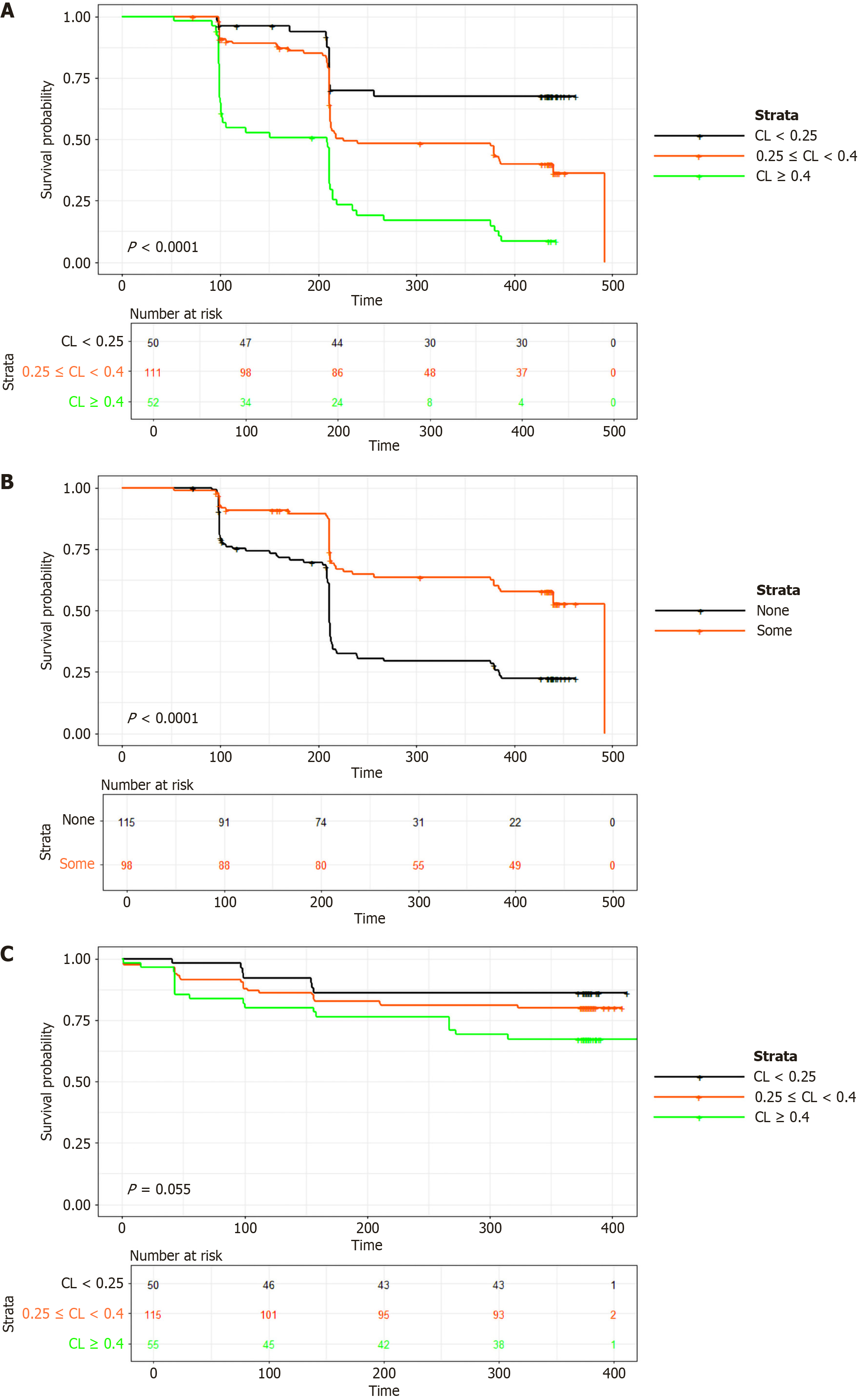
Figure 3 Kaplan-Meier curves for the time to first onset of anti-drug antibodies and drug survival based on initial infliximab clearance.
A: Effect of clearance on time to development of anti-drug antibodies. Black line: Low clearance, orange line: Medium clearance and green line: Fast clearance; B: Effect of use of immunomodulators on time to development of antidrug antibodies. Orange line: Some or no immunomodulator used, black line: No immunomodulator used; C: Effect of clearance on drug survival. Black line: Low clearance, orange line: Medium clearance, green line: Fast clearance.
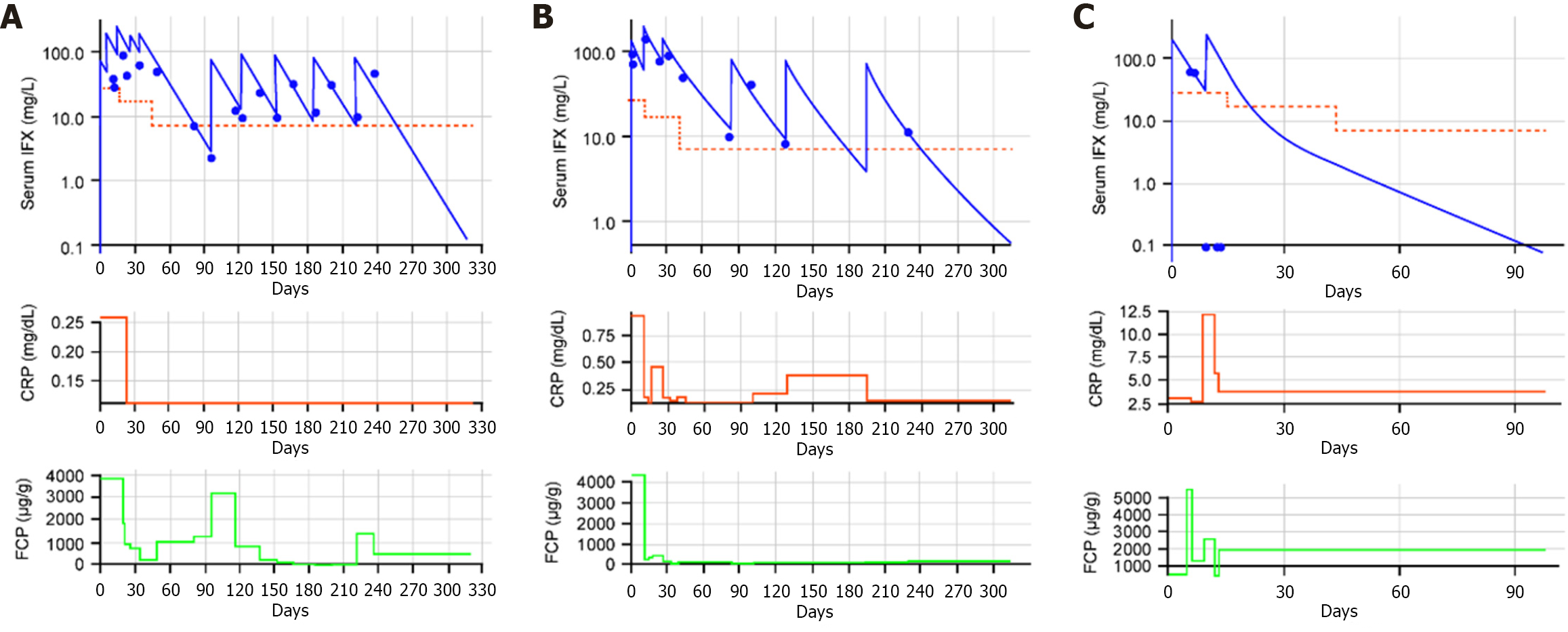
Figure 4 Observed vs predicted serum infliximab concentrations and C-reactive protein and fecal calprotectin levels.
A: Patient 3; B: Patient 4; C: Patient 9. In each figure panel: Serum infliximab (IFX) concentrations, solid lines show individual predicted values, filled circles show observed concentrations, and dashed lines shows the target threshold. CRP: C-reactive protein; FCP: Fecal calprotectin.
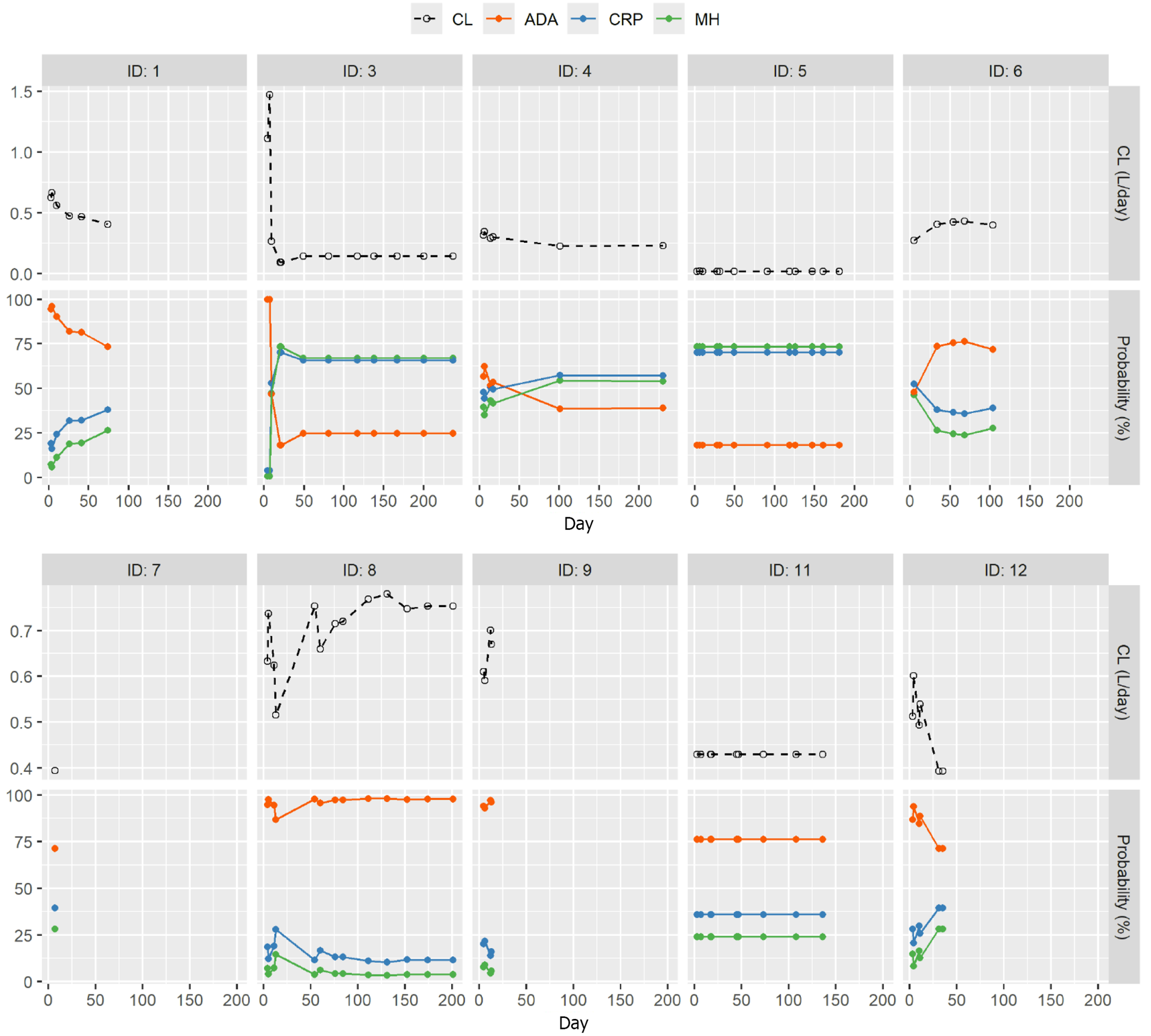
Figure 5 Observed individual clearance (upper panel) and probability of mucosal healing, C-reactive protein normalization and antidrug antibody formation (lower panels) over time by ID.
The upper panels are individual clearance (CL) over time and the lower panels are individual probabilities of antidrug antibody (ADA), C-reactive protein (CRP) normalization, and mucosal healing (MH).
- Citation: Mould DR, Kutschera M, Primas C, Reinisch S, Novacek G, Lichtenberger C, Dervieux T, Bae Y, Lee SH, Lee JH, Reinisch W. Identification of strong correlations of clearance with clinical outcomes for inflammatory bowel disease. World J Gastroenterol 2026; 32(5): 113505
- URL: https://www.wjgnet.com/1007-9327/full/v32/i5/113505.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i5.113505