©The Author(s) 2026.
World J Gastroenterol. Jan 21, 2026; 32(3): 112437
Published online Jan 21, 2026. doi: 10.3748/wjg.v32.i3.112437
Published online Jan 21, 2026. doi: 10.3748/wjg.v32.i3.112437
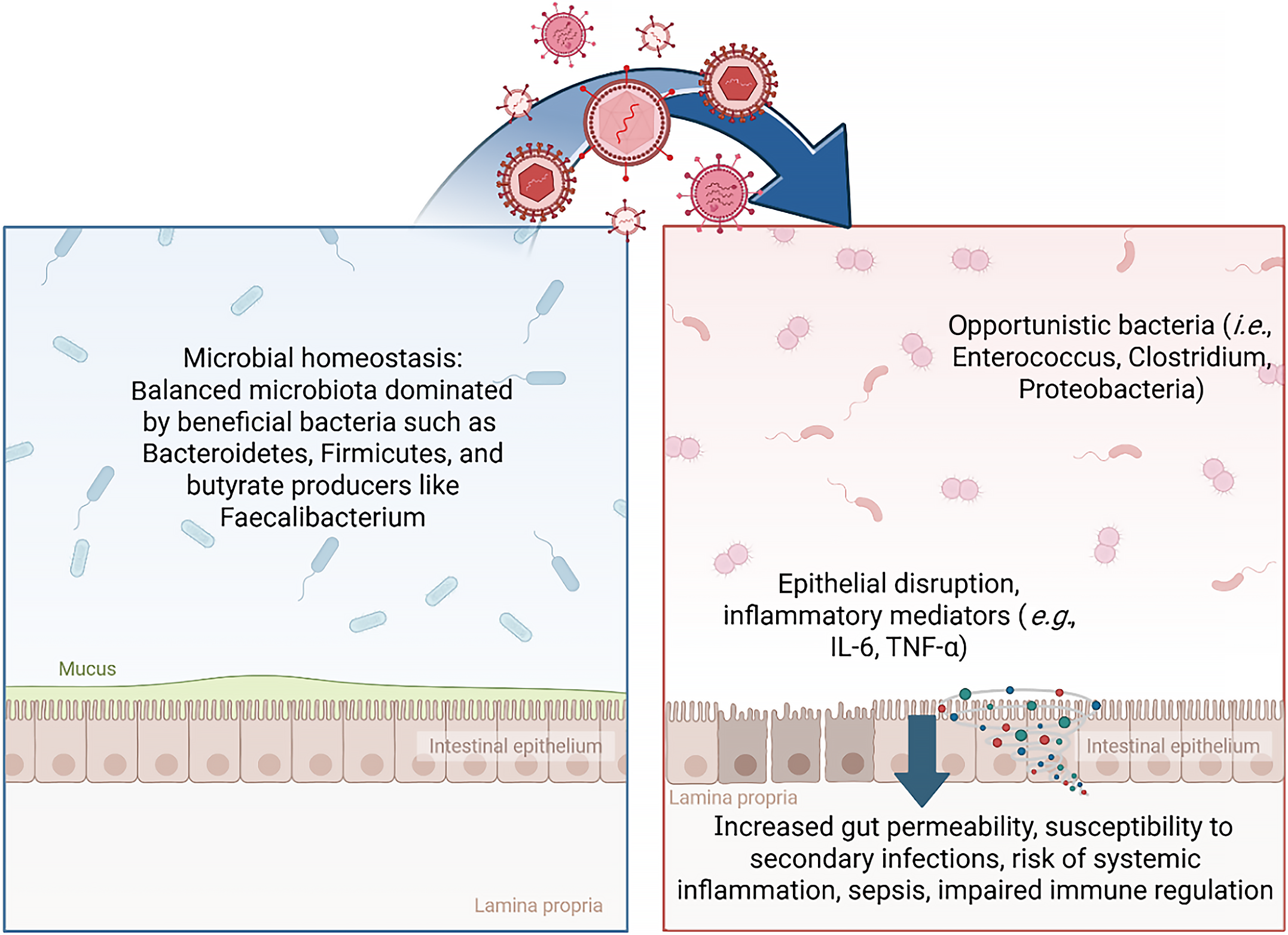
Figure 1 In homeostasis, the gut microbiota is balanced, dominated by beneficial bacteria such as Bacteroidetes, Firmicutes, and butyrate producers like Faecalibacterium.
Viral infection can promote dysbiosis, characterized by expansion of opportunistic bacteria (e.g., Enterococcus, Clostridium, Proteobacteria), disruption of the intestinal epithelium, and release of inflammatory mediators (e.g., interleukin-6, tumor necrosis factor-α). These changes increase gut permeability and susceptibility to systemic inflammation, sepsis, and impaired immune regulation. Created in BioRender (Supplementary material). IL: Interleukin; TNF: Tumor necrosis factor.
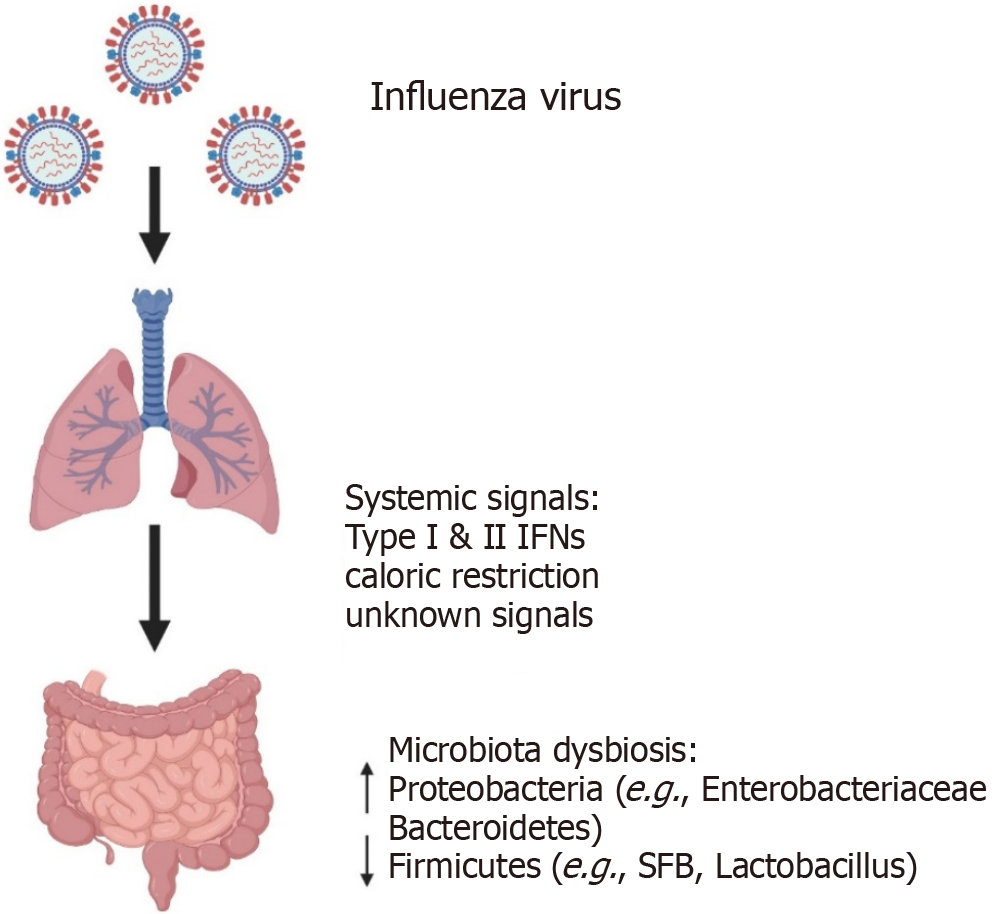
Figure 2 Influenza-induced gut microbiome dysbiosis in mice.
Acute influenza infection in the respiratory tract alters gut bacterial composition without direct gastrointestinal viral replication, suggesting regulation via systemic immune or physiological signals (e.g., type I/II interferons, caloric restriction). Observed microbial changes include increased Proteobacteria (e.g., Enterobacteriaceae) and Bacteroidetes, and reduced Firmicutes (e.g., segmented filamentous bacteria, Lactobacillus). Studies have primarily focused on specific pathogen-free-housed laboratory mice. Therefore, the relevance to humans or wild mammals with diverse microbiomes remains uncertain. Created in BioRender (Supplementary material). IFNs: Interferons; SFB: Segmented filamentous bacteria.
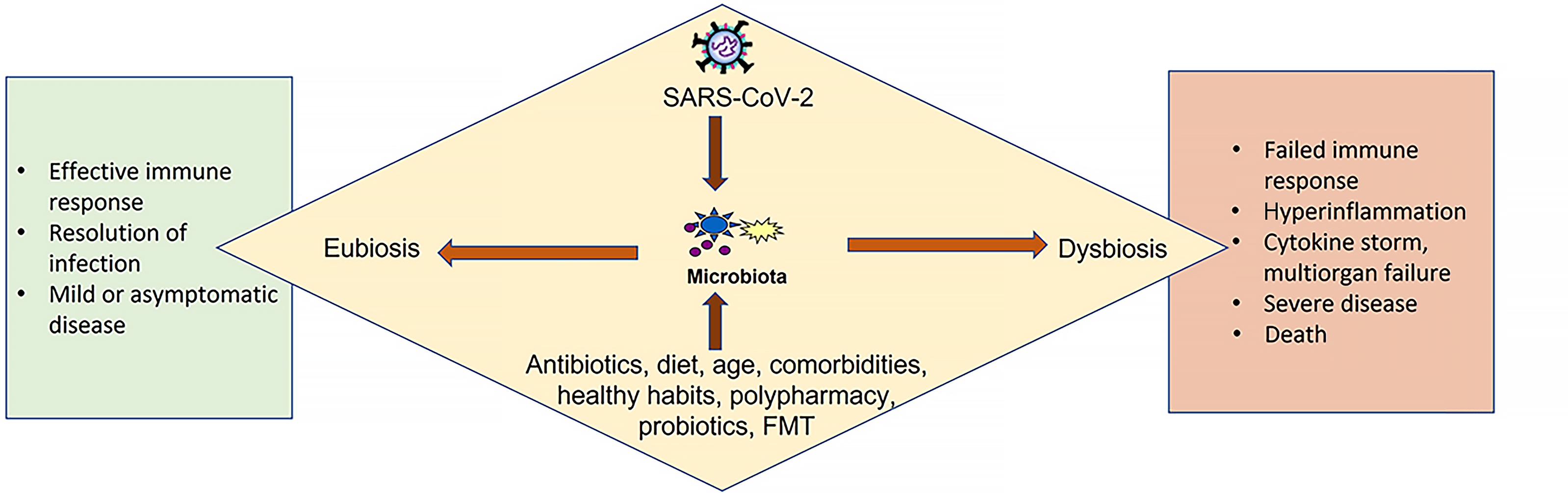
Figure 3 Severe acute respiratory syndrome coronavirus 2 interacts with the gut microbiota, influencing host outcomes.
A preserved balanced microbiota supports effective immune responses, infection resolution, and mild or asymptomatic disease (eubiosis). In contrast, disruption of the microbiota (dysbiosis) contributes to failed immune responses, hyperinflammation, cytokine storm, multiorgan failure, and severe disease or death. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; FMT: Fecal microbiota transplantation.
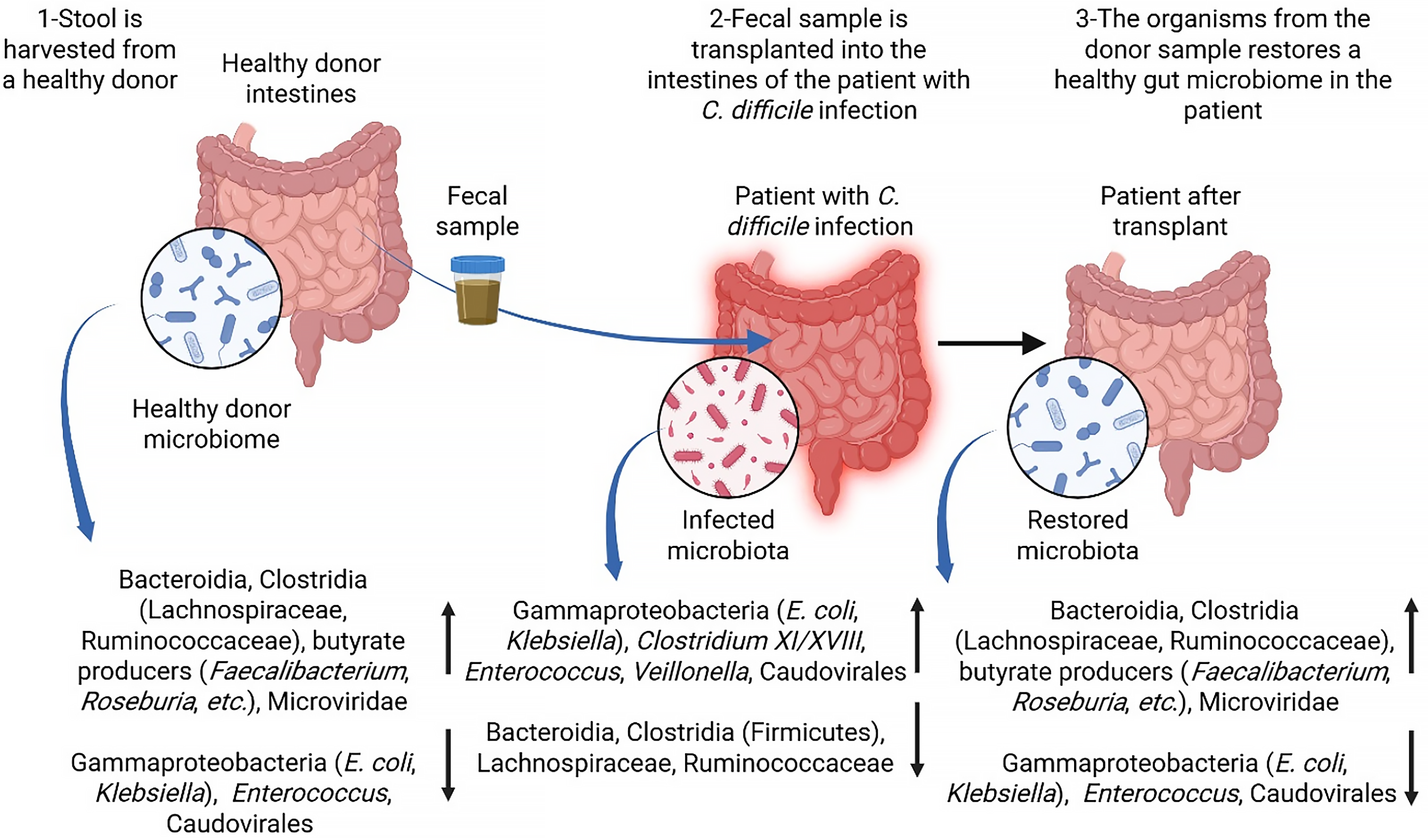
Figure 4 Fecal microbiota transplantation for Clostridioides difficile infection.
The process and effect of fecal microbiota transplantation in patients with Clostridioides difficile infection are presented. In step 1, stool containing a healthy microbial community is harvested from a healthy donor. The donor microbiota includes Bacteroidia, Clostridia (Lachnospiraceae, Ruminococcaceae), butyrate producers (Faecalibacterium, Roseburia), and Microviridae, along with low levels of potential pathogens such as Escherichia coli, Klebsiella, and Enterococcus. In step 2, the donor fecal sample is transplanted into the intestine of a patient with disrupted gut microbiota resulting from a Clostridioides difficile infection. The infected microbiome is characterized by elevated levels of Gammaproteobacteria (Escherichia coli, Klebsiella), Clostridium XI/XVIII, Enterococcus, Veillonella, and Caudovirales. In step 3, following transplantation, the patient’s gut microbiome is restored to a composition resembling that of a healthy donor, with the re-establishment of beneficial bacteria and a reduction in pathogenic species, thereby promoting recovery and preventing the recurrence of Clostridioides difficile infection. Created in BioRender (Supplementary material). E. coli: Escherichia coli; C. difficile: Clostridioides difficile.
- Citation: Velikova T, Ali H, Batselova H, Chervenkov L, Miteva D, Peruhova M, Gulinac M, Tomov L, Mitova-Mineva Y, Velev V. Interplay between viral infections and gut microbiota dysbiosis: Mechanisms and therapeutic potential. World J Gastroenterol 2026; 32(3): 112437
- URL: https://www.wjgnet.com/1007-9327/full/v32/i3/112437.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i3.112437