©The Author(s) 2026.
World J Gastroenterol. Jan 7, 2026; 32(1): 114479
Published online Jan 7, 2026. doi: 10.3748/wjg.v32.i1.114479
Published online Jan 7, 2026. doi: 10.3748/wjg.v32.i1.114479
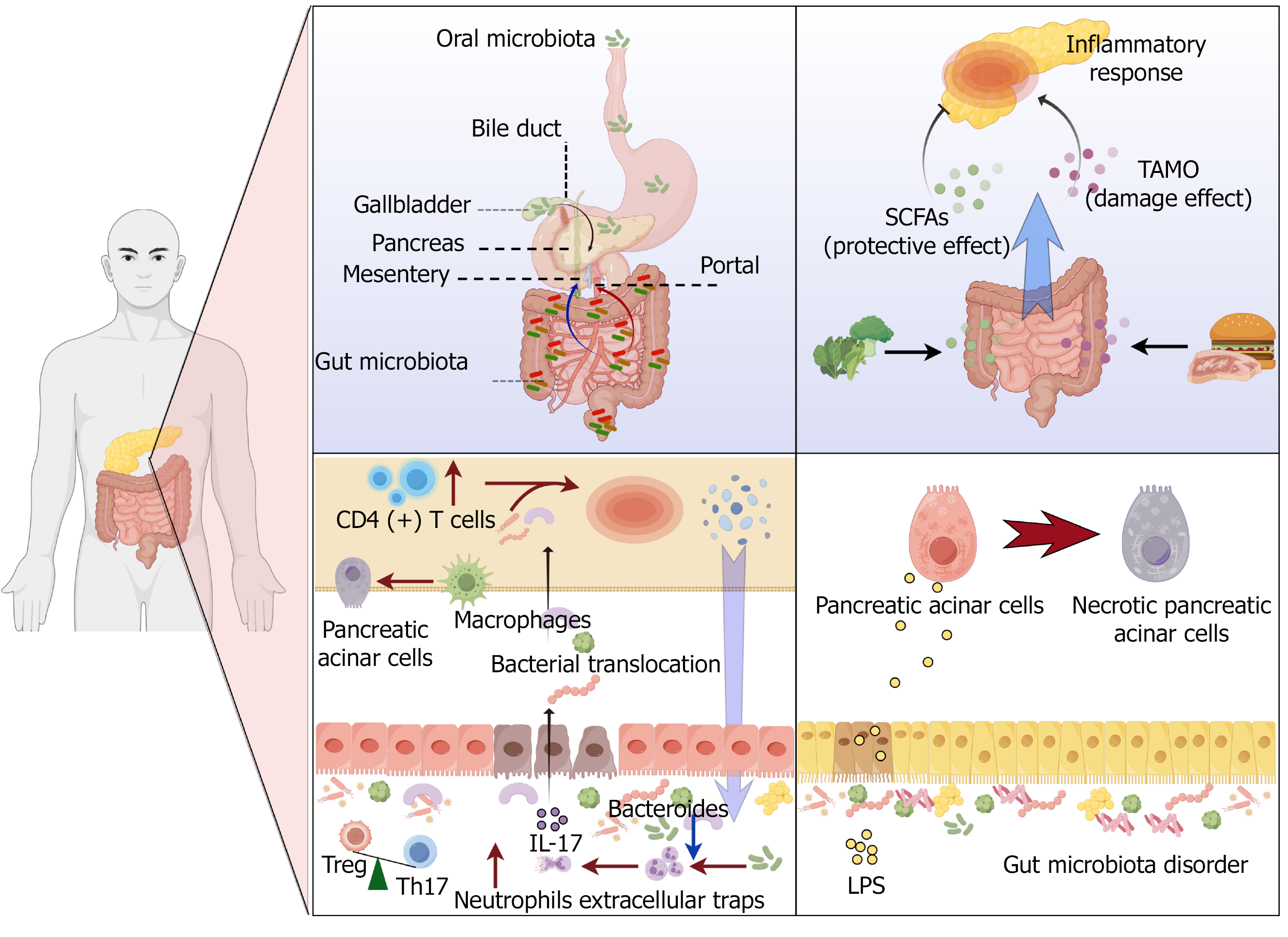
Figure 1 Bidirectional communication between the gut and pancreas.
Gut microbiota may enter the pancreas through: Portal circulation; Mesenteric lymph nodes; Pancreatic duct reflux. Gut microbiota metabolites mediate bidirectional communication between the gut and pancreas. Gut microbiota can cause bacterial translocation by regulating the regulatory T cell/T helper 17 cell ratio. The reduction of Bacteroides abundance can regulate neutrophils to form neutrophil extracellular traps, leading to impairment of intestinal barrier function. The inflammatory response of the pancreas leads to a decrease in antimicrobial peptide synthesis, which results in a disturbance of the gut microbiota. Gut microbiota disorders lead to lipopolysaccharide elevation, leading to pancreatic acinar cell damage. IL: Interleukin; Treg: Regulatory T; Th: T helper; CD: Cluster of differentiation; SCFAs: Short-chain fatty acids; TMAO: Trimethylamine N-oxide; LPS: Lipopolysaccharide.
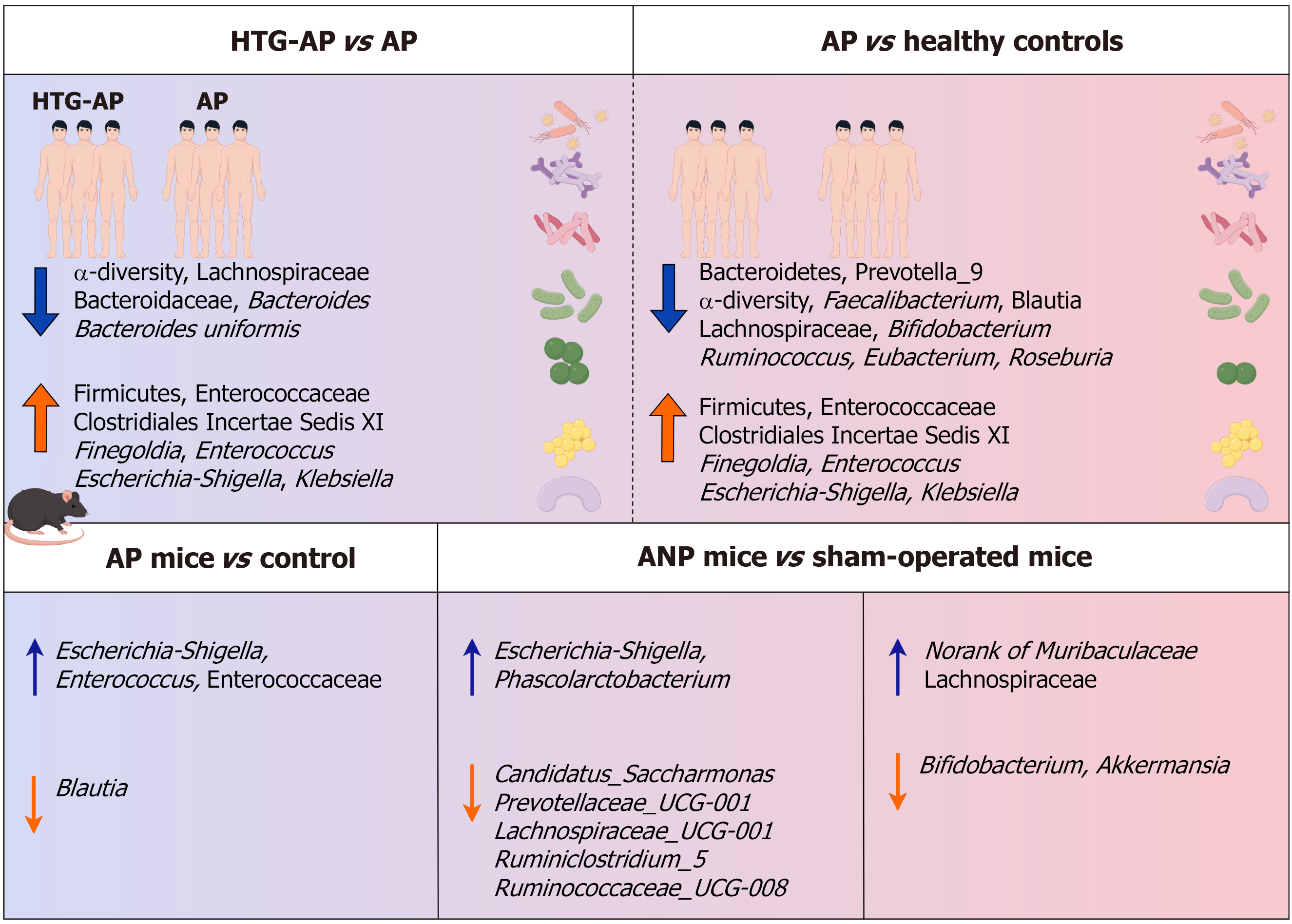
Figure 2 Changes in gut microbiota composition in hypertriglyceridemia-induced acute pancreatitis and acute pancreatitis.
Clinically, it has been observed that acute pancreatitis (AP) can lead to changes in the composition and function of gut microbiota, and the gut microbiota of patients with hypertriglyceridemia-induced AP shows different dominant bacteria from that of AP. AP: Acute pancreatitis; HTG: Hypertriglyceridemia; ANP: Acute necrotizing pancreatitis.
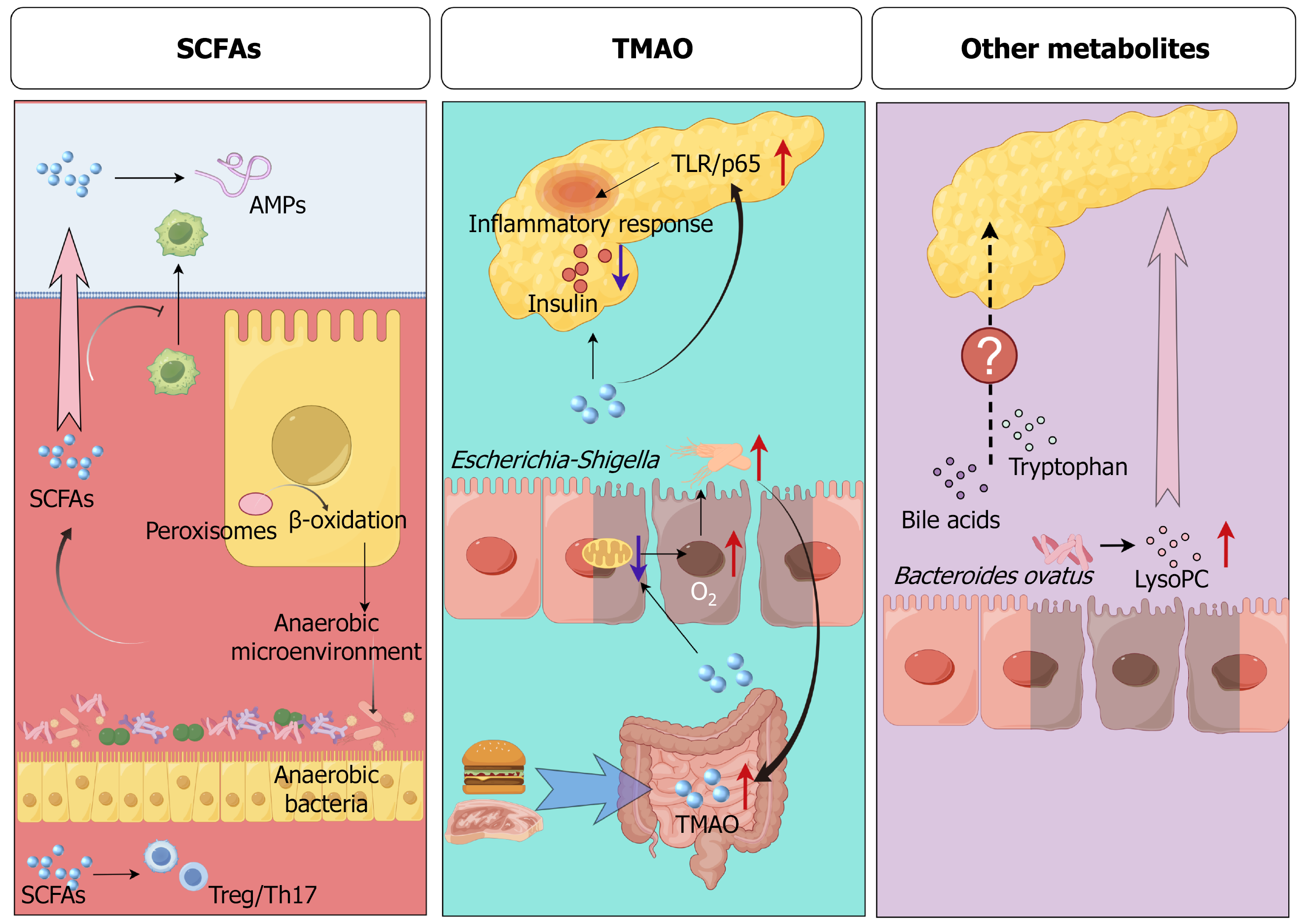
Figure 3 Gut microbiota-driven metabolites regulate the inflammatory response of the pancreas.
Short-chain fatty acids (SCFAs) can regulate the regulatory T cell/T helper 17 cell ratio and the production of antimicrobial peptides in the pancreas, and inhibit the infiltration of macrophages into the pancreas. In addition, SCFAs can activate peroxisomes, promote colonic epithelial cells toward β-oxidation, and deplete gut oxygen to maintain an anaerobic environment and inhibit the overgrowth of pathogenic bacteria. Trimethylamine N-oxide (TMAO) impairs mitochondrial function in colonic epithelial cells, resulting in an increase in gut oxygen concentration and promoting the growth of Escherichia-Shigella. In addition, TMAO also leads to decreased insulin secretion and aggravates pancreatic inflammation through Toll-like receptor/p65. Bacteroides ovatus in the gut can synthesize lysophosphatidylcholine to promote inflammation of the pancreas. SCFAs: Short-chain fatty acids; TMAO: Trimethylamine N-oxide; AMP: Antimicrobial peptides; Treg: Regulatory T; Th: T helper; TLR: Toll-like receptor; LysoPC: Ly
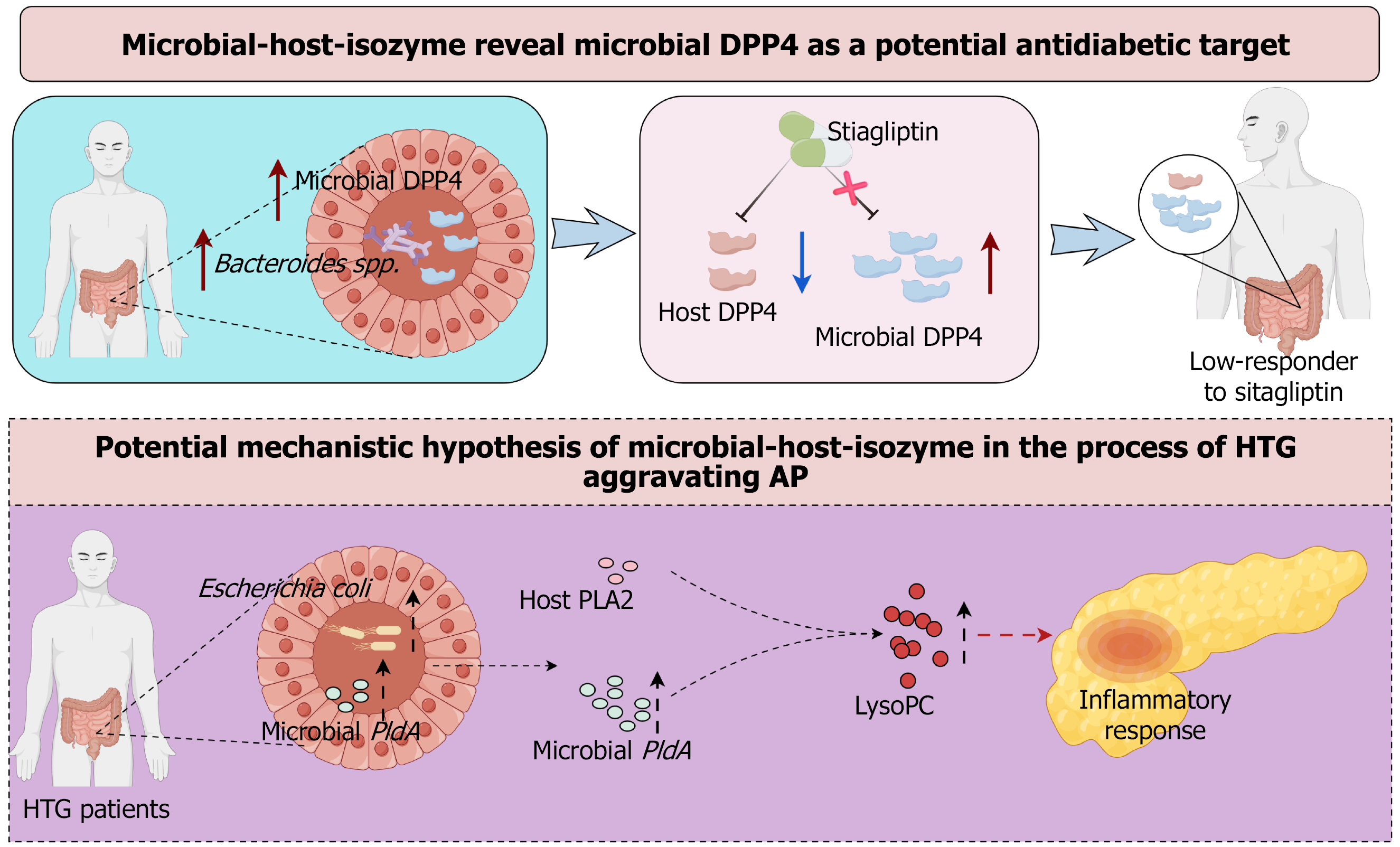
Figure 4 Future prospects of microbial-host-isozyme in the diagnosis and treatment of hypertriglyceridemia-induced acute pancreatitis.
Bacteroides spp. in the gut can secrete dipeptidyl peptidase 4 (DPP4). Sitagliptin can only inhibit host DPP4 activity but not microbial-derived DPP4 activity, leading to a low response to sitagliptin in patients. Escherichia coli (E. coli) is frequently enriched in patients with hypertriglyceridemia. PldA of E. coli can synthesize products with phospholipase A2 activity to promote the production of lysophosphatidylcholine and thus aggravate the inflammatory response of acute pancreatitis. DPP4: Dipeptidyl peptidase 4; AP: Acute pancreatitis; HTG: Hypertriglyceridemia; PLA2: Phospholipase A2; LysoPC: Lysophosphatidylcholine.
- Citation: Song XF, Liu Y, Fei QM, Xu CL, Ji FP. Potential influence of gut microbiota on the process of hypertriglyceridemia-aggravated acute pancreatitis. World J Gastroenterol 2026; 32(1): 114479
- URL: https://www.wjgnet.com/1007-9327/full/v32/i1/114479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i1.114479