Published online Jan 7, 2026. doi: 10.3748/wjg.v32.i1.114558
Revised: October 30, 2025
Accepted: November 19, 2025
Published online: January 7, 2026
Processing time: 104 Days and 14.9 Hours
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by clinical symptoms of diarrhea and mucopurulent bloody stools, and its incidence is increasing globally. The etiology and pathogenesis of UC remain elusive. Cur
Core Tip: As a traditional Chinese medicine, Scutellaria baicalensis Georgi (HQ) plays a crucial role in the treatment of ulcerative colitis (UC) since it contains multiple active components, such as baicalein, wogonin, baicalin, wogonoside and polysaccharides. HQ exerts therapeutic efficacy in UC through multiple mechanisms, including modulation of pro- and anti-inflammatory cytokine expression, regulation of immune cell function, protection of the intestinal barrier, and exertion of antioxidant and anti-apoptotic activities, as well as modulation of the gut microbiota. Clinically, HQ-based formulations, either used alone or in combination with conventional Western medications, have demonstrated favorable outcomes in UC management, such as reducing adverse drug reactions, enhancing therapeutic efficacy, and lowering recurrence rates.
- Citation: Ding Y, Wang CY, Pan YT, Wang YJ, Zhao AG, Wen HZ. Scutellaria baicalensis Georgi as a potential therapeutic drug intervention in ulcerative colitis: Mechanisms of action and clinical trials. World J Gastroenterol 2026; 32(1): 114558
- URL: https://www.wjgnet.com/1007-9327/full/v32/i1/114558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i1.114558
Ulcerative colitis (UC) is a chronic and recurrent inflammatory bowel disease. Its pathogenesis is complex, with inflammation typically originating in the rectum and progressively extending to part or all the colon. Its clinical manifestations primarily include diarrhoea, mucopurulent bloody stools, abdominal pain, and tenesmus[1]. Epidemiological evidence indicates that the global prevalence of UC reached approximately 5 million individuals in 2023, with its incidence showing an annual upward trend, and particularly, the incidence of UC is projected to increase four-folds across Asia by 2035, making UC as a significant global health challenge[2]. Recently, common treatment options for UC include 5-aminosalicylic acid (5-ASA), glucocorticoids, immunosuppressants, biologics, and small-molecule drugs. However, these medications may increase the risk of adverse effects, such as thrombosis and infection[3].
In traditional Chinese medicine (TCM), some classifications, such as “Chang Pi”, “Li Ji”, and “Jiu Li”, align with the symptoms of UC, such as diarrhoea with mucus and blood, along with the disease’s chronic course and tendency to recur. Regarding pathogenesis, TCM holds that UC arises from the combined effects of underlying deficiency and superficial excess. Patients often exhibit constitutional spleen qi deficiency, which is exacerbated by exposure to external pathogens or dietary indiscretion. During the active phase of UC, its pathogenesis primarily involves damp-heat obstructing the intestines, and TCM treatment is to clear heat, resolve toxins, and transform dampness. During the remission phase of UC, it often presents mixed deficiency-excess patterns, primarily due to impaired spleen function and stagnant damp turbidity, and the TCM treatment is to tonify qi, strengthen the spleen, consolidate the kidneys, while concurrently clearing heat and transforming dampness[4]. Notably, TCM has long been used in folk medicine as adjunctive therapies for UC. For instance, many Chinese herbal formulas[5-7] have been clinically shown to alleviate symptoms including diarrhea and hematochezia, modulate intestinal immune responses, and promote mucosal healing. In recent years, TCM has emerged as an important therapeutic modality for UC, and its multi-targeted, multi-level, and multi-pathway characteristics have been progressively validated[8]. Furthermore, TCM demonstrates distinct advantages in alleviating symptoms, controlling disease progression, and mitigating the toxic side effects of Western pharmaceuticals[9-11].
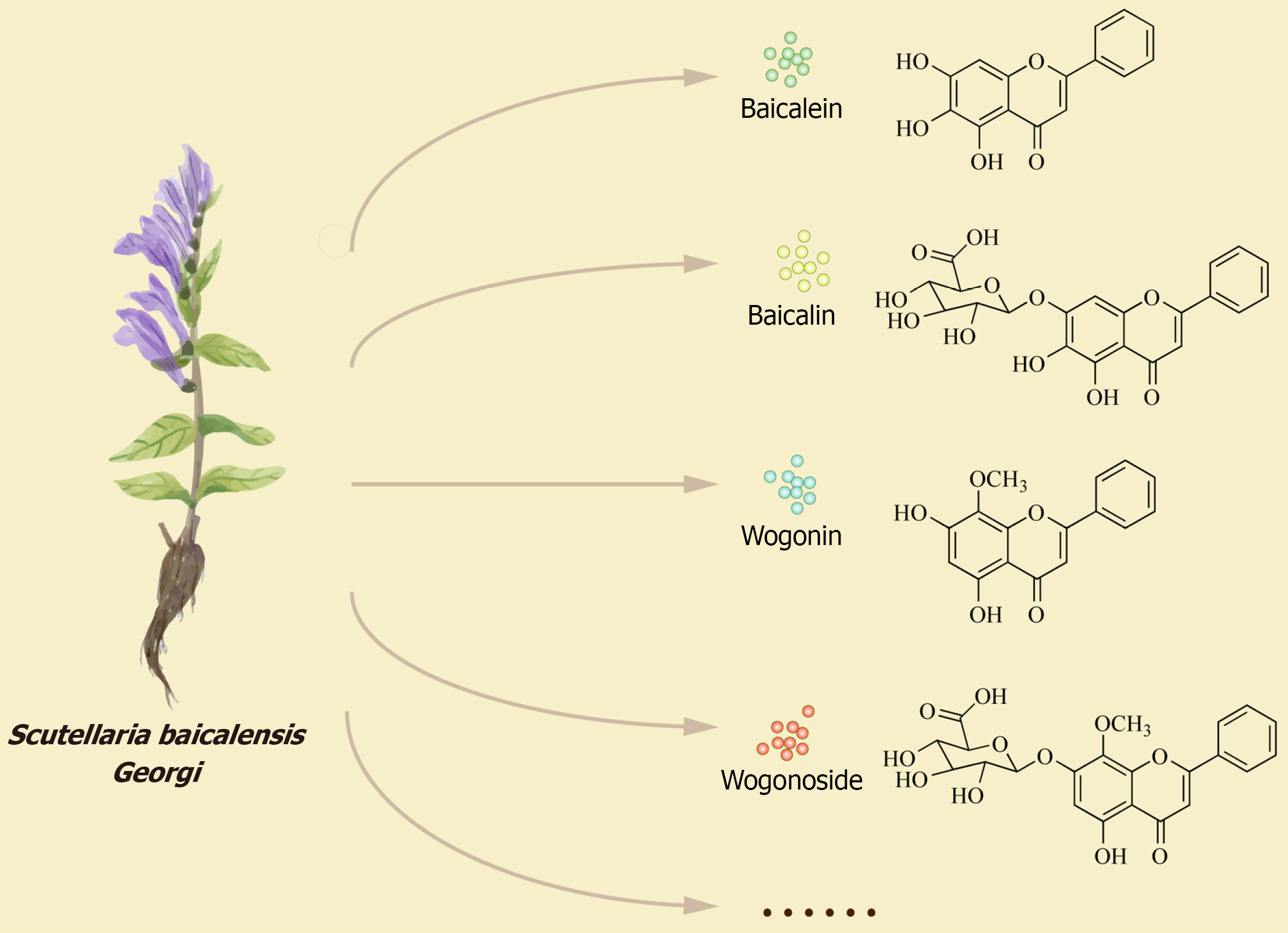
Scutellaria baicalensis Georgi (Huangqin, HQ), the dried root of Scutellaria L. (family Labiaceae), demonstrates anti-inflammatory (corresponding to heat-clearing and fire-reducing), diuretic (dampness-eliminating), antioxidant, deto
To date, 132 compounds have been isolated from HQ, including flavonoids, phenethyl alcohol glycosides, sterols, polysaccharides, terpenoids, amides, and phenolic compounds. There are a total of 56 flavonoids, including 42 flavones, 3 flavanols, 9 flavanones, 1 chalcone, and 1 biflavone, with baicalein and wogonin being the most abundant; and there are 44 flavonoid glycosides, with baicalin and wogonoside being the most abundant[17,18]. Flavonoids are the most bioactive extracts of HQ, with baicalein, wogonin, baicalin, and wogonoside being the principal active components responsible for its anti-inflammatory effects[19,20] (Figure 1). Baicalein, wogonin, and their glycoside derivatives can directly affect immune cells (such as lymphocytes, macrophages, monocytes, neutrophils, dendritic cells, and mast cells) by modulating the mitogen-activated protein kinase (MAPK), peroxisome proliferator-activated receptor, phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), nuclear factor kappa-B (NF-κB), and Janus kinase/signal transducer and activator of transcription signaling pathways, thereby inhibiting the production of pro-inflammatory cytokines and other inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, IL-8, nitric oxide, prostaglandins, and reactive oxygen species (ROS)[21,22]. Furthermore, the active components of HQ exhibit other functions, such as regulation of T helper cell (Th) 1/Th2 balance[23], anti-tumor effects[24-26], anti-bacterial activity[27-29], and anti-viral activities[30] (Table 1). Throughout history, HQ has consistently played a significant role in the treatment of UC. Therefore, to provide valuable reference for future basic research and clinical applications of HQ, this review summarizes the mechanisms of action and clinical research progress of HQ in treating UC through a literature search.
| Ingredients | Pharmacological activities | Experimental type | Specific model | Main findings | Ref. |
| Huangqin | Anti-inflammatory | In vivo and in vitro | DNP IgE-induced rats and rat, peritoneal mast cells, human mast cells | Suppressing passive cutaneous anaphylactic reactions and reduces histamine release in rat peritoneal mast cells. In human mast cell-1 cells, it restores IL-8 and TNF-α expression while inhibiting MAP kinase expression induced by compound 48/80 | [21] |
| Baicalein | In vivo and in vitro | Mouse macrophage cell line RAW264.7, male Balb/c mice | Inhibiting the increase of RAW264.7 viability. Inhibiting the production of NO through downregulating iNOS without affecting the enzyme activity. Inhibiting intracellular ROS stimulated by LPS, whereas augmented intracellular SOD level in RAW264.7 cells. Inhibiting the production of inflammatory mediators induced by LPS in RAW264.7 cells. Decreasing the level of serum nitrite accumulation and TNF-α | [21,22] | |
| Baicalin | In vitro | HPS SH0165 strain and piglet’s mononuclear phagocytes, high-fat diet-fed rat | Inducing ROS production, arresting the cell cycle and promoting apoptosis via the PKC-MAPK signaling pathway in piglet’s mononuclear phagocytes. Reducing ROS production, suppression of cleavage of caspase-3 in inducing apoptosis, and inhibition of activation of the PKC-MAPK signaling pathway for down-regulating p-JNK, p-p38, p-ERK, p-PKC-α and PKC-δ in PMNP triggered by HPS. Targeting the liver AMPK pathway to benefit obesity-related diseases and hepatic steatosis | ||
| Wogonin | In vitro | Mouse macrophage cell line RAW264.7 | Restoring the viability of dsRNA (polyinosinic-polycytidylic acid)-induced RAW 264.7 mouse macrophages. Inhibiting the production of oxide, IL-1α, IL-1β, IL-6, IL-10, IP-10, G-CSF, GM-CSF, LIF (IL-6-like cytokine), LIX/CXCL5, MCP-1, M-CSF, MIP-1α, MIP-1β, MIP-2, RANTES/CCL5, TNF-α, and VEGF production. Reducing calcium release and mRNA expression of STAT1 and STAT3 in dsRNA-induced RAW 264.7 cells | [21] | |
| Wogonoside | In vivo | Lipopolysaccharide/D-galactosamine-induced mice | Inhibiting the production of serum alanine transaminase, aspartate aminotransferase, IL-1β, TNF-α, and hepatic malondialdehyde content induced by LPS/GalN. Promoting the expression of Nrf2, NQO-1, GCLC, and HO-1. Reducing expression of hepatic NLRP3, ASC, caspase-1, and IL-1β induced by LPS/GalN | [21] | |
| Oroxylin A | In vivo and in vitro | C57BL/6 mice, BALB/C mice, H460, Jurkat and Lewis cell lines | Inhibiting the generation of Tregs in lung cancer environment by inhibiting the T cells’ response to TGF-β1, decreasing the secretion of TGF-β1 in lung cancer cells via NF-κB signaling | [21] | |
| Baicalein | Regulation of Th1/Th2 balance | In vivo | OVA-induced murine asthma model | Regulating the balance of Th1/Th2 cytokines by suppressing the development of airway inflammation via shifting from a Th2 to Th1 response in the OVA-induced asthma | [23] |
| Wogonin | |||||
| Scutellaria baicalensis ethanol extract | |||||
| Baicalein | Anti-tumor | In vitro and in vivo | Human colorectal cancer HCT116 cells and AOM/DSS-induced ICR mice | Reducing the viability of HCT116 cells. Inducing apoptosis in HCT116 cells. Suppressing the NF-κB activity through the PPARγ activation in HCT116 cells. Suppressing migration in HCT116 cells. Inhibiting AOM/DSS-induced colitis and tumorigenesis | [24] |
| Wogonin | In vitro and in vivo | A549 cells and A427 cells, human HCC cell line (HepG2), human normal liver cell line (LO2), human hepatocellular carcinoma MHCC97 L and MHCC97 L_luciferase lines, orthotopically HCC-implantation mice model | Inhibiting A549 and A427 lung cancer cell viability without affecting BEAS-2B normal cells. Reducing A427 cell count. Inducing apoptosis of A427 cells. Increasing the expression of caspases 8/9/3 in A427 cells. Inducing ROS generation in A427 cells. Inducing autophagy in A427 cells. Inhibiting cell proliferation and inducing G1 arrest in HCC cells. Inducing cyclin D1 phosphorylation on T286 site and inducing nuclear export in MHCC97 L cells. Promoting G1 arrest of HCC cells through activation of GSK3beta. Suppressing liver tumor growth in vivo | [25,26] | |
| Baicalein | Anti-bacterial | In vitro and in vivo | Escherichia coli cells, clinically isolated and cultured multidrug-resistant strains of Helicobacter pylori, Aeromonas hydrophila and grass carp model | Inhibiting the activity of Escherichia coli. Inhibiting the activity of multidrug-resistant strains of Helicobacter pylori. Inhibiting the activity, biofilm formation and motility of Aeromonas hydrophila | [27-29] |
| Baicalin | |||||
| Baicalein | Anti-viral | Network pharmacology | Regulating ACE2 by acting on the RAS pathway. Inhibiting the binding of 2019-nCoV to ACE2 to control the progression of COVID-19 | [30] | |
| Wogonin |
Our search was conducted using databases China National Knowledge Infrastructure, Wanfang Data, SinoMed, PubMed, Web of Science, and Scopus, with keywords including “Scutellaria baicalensis Georgi”, “S. baicalensis ethanol extract”, “Huangqin”, “baicalein”, “baicalin”, “wogonin”, “wogonoside”, “Oroxylin A”, “Ulcerative Colitis”, and “Inflammatory Bowel Disease”.
UC is a multifactorial disease influenced by genetic predisposition, environmental exposures, and lifestyle factors[31]. Although its precise pathogenesis remains incompletely understood, UC is widely recognized as the result of a complex interplay among genetic susceptibility, immune dysregulation, gut microbiota alterations, and environmental triggers. Among these factors, immune imbalance[32], intestinal microbiota dysbiosis[33], and disruption of the intestinal mucosal barrier[34] represent the most intensively studied aspects of UC pathogenesis, as well as the most consistently reported and therapeutically targeted processes in both preclinical studies and clinical trials involving HQ and HQ-based formulas.
The immune system primarily comprises innate immunity and adaptive immunity, both of which play crucial roles in the pathogenesis of UC[35,36]. Innate immune cells include neutrophils, macrophages, monocytes, innate lymphoid cells, and dendritic cells, while adaptive immune cells include cytotoxic T cells, regulatory T cells (Tregs), and Th cells, such as Th1/Th2, Th9, Th17, and Th22. These cells are closely associated with the onset and progression of UC. For instance, imbalances in Th1/Th2 or Th17/Treg ratios may drive excessive inflammatory responses[37]. When macrophages polarize towards the pro-inflammatory M1 phenotype, an increased M1/M2 macrophage ratio may promote UC progression[38,39]. Neutrophils, one of the earliest inflammatory cells involved in the onset of UC, have been considered an indicator of therapeutic efficacy for the resolution of neutrophil-related inflammation of the intestinal mucosa[40].
Under normal conditions, Th1 and Th2 cells, pro- and anti-inflammatory cytokines maintain a dynamic equilibrium to preserve intestinal homeostasis. UC patients exhibit Th2 dominance, with the percentage of peripheral blood Th17 cells markedly higher than that in healthy individuals[41,42]. Pro-inflammatory cytokines, such as IL-1, TNF-α, IL-6, IL-8, and IL-18, are highly expressed in UC patients, while anti-inflammatory cytokines (such as IL-4 and IL-10) are downregulated[43-47]. Massive inflammatory cell infiltration and elevated pro-inflammatory cytokines in the gut induce necrosis and sloughing of intestinal epithelial cells, which may cause damage to the intestinal mucosal barrier and lead to the onset and progression of UC.
Besides, studies have shown that multiple factors, including microbes, pathogen-associated molecular patterns, medications, chemical toxins, lifestyle, and genetics, contribute to immune dysregulation in UC. Dysbiosis of the intes
Recent studies have demonstrated a close association between UC pathogenesis and intestinal microecological imbalance. The gut microbiota, with its dynamic diversity and abundance, plays a crucial role in regulating mucosal immunity[53]. Based on physiological function, gut microbiota can be categorized into three groups, including commensal bacteria, pathogenic bacteria, and opportunistic pathogens. Beneficial bacteria include the phyla Firmicutes, Bacteroidetes, Lactobacillus, and Bifidobacterium, and they play a crucial role in improving intestinal function[54,55]. Conversely, pathogenic bacteria, such as Clostridium difficile, readily induce inflammatory responses in the intestine[56].
Microbiome dysbiosis in UC patients primarily manifests as alterations in microbial abundance, microbial diversity, and metabolite levels[57]. UC patients exhibit reduced Firmicutes abundance but increased abundance of Proteobacteria and Fusobacteria[58]. Additionally, studies reveal significantly reduced abundance of Bifidobacteria and Lactobacillus in patients with active UC compared to healthy controls, along with markedly elevated Escherichia coli abundance[59].
The imbalance of the intestinal microbiota in patients with UC is closely associated with factors such as inappropriate antibiotic use, dietary pattern alterations, psychological stress, sleep disturbances, environmental pollution, abnormal gastrointestinal motility, and dysregulated bile acid metabolism[60]. Studies have shown that active UC is often accom
The intestinal mucosal barrier primarily comprises four components: Mechanical, biological, immunological, and chemical barriers, which collectively serve as a line of defense against the entry of harmful substances. In patients with active UC, the number of colonic goblet cells is reduced and the mucosal barrier is compromised, indicating that mucosal barrier dysfunction is a key driver of the disease[64]. Damage to the intestinal mucosal barrier is closely associated with alterations in tight junction (TJ) proteins, mucus thinning, epithelial injury, apoptosis, stress responses, as well as exposure to non-steroidal anti-inflammatory drugs and alcohol, among other factors[65]. TJ proteins primarily comprise three types of membrane proteins: Claudins, occludins, and junctional adhesion molecules. The expression of these proteins serves as a key indicator for assessing intestinal mucosal barrier function in UC. Tan et al[66] found that downregulated expression of TJ proteins at inflammatory sites of the intestinal mucosa is closely correlated with mucosal healing in 80 UC patients. Du et al[67] found enlarged intercellular spaces and elevated permeability in the intestinal mucosa of patients with active UC. TJ proteins can be regulated through the myosin light chain kinase/myosin light chain 2 pathway.
Current management of UC follows a severity-stratified approach. For mild-to-moderate UC, 5-ASAs are first-line therapy, with the formulation (oral, suppository, or enema) tailored to disease extent. Corticosteroids, such as budesonide, are used when 5-ASA therapy is insufficient. In moderate-to-severe UC, advanced therapies, including anti-TNF agents (infliximab, adalimumab, golimumab), vedolizumab, ustekinumab, or tofacitinib, are indicated. Acute severe UC requires hospitalization and intravenous steroid therapy, with non-responders receiving rescue therapy with infliximab or cyclosporin. Colectomy is considered for medically refractory cases or when complications develop. The primary treatment goals are to achieve both clinical and endoscopic remission[31,68].
However, the long-term use of conventional therapies is often limited by their adverse effects. TCM with its multi-target regulatory properties and low toxicity, offers a promising complementary strategy and is increasingly applied in the management of UC[69]. HQ is a key component in several patented formulations, including Xilei San, Fufangkushen, and Qingchang Huashi recipe[70]. Compared with other herbs used in UC treatment, such as Astragalus (astragalus polysaccharides), Coptidis Rhizoma (berberine), or Epimedium (total flavonoids), HQ is distinguished by its high content of specific flavones, such as baicalein, which potently inhibit pro-inflammatory cytokines (e.g., TNF-α, IL-6) and enhance intestinal barrier function. While other herbs, such as Sophora japonica, contribute to mucosal repair and resveratrol from Polygonum cuspidatum modulates the NF-κB signaling pathway, HQ demonstrates a more comprehensive and synergistic pharmacological profile, targeting multiple inflammatory and immune pathways simultaneously[71].
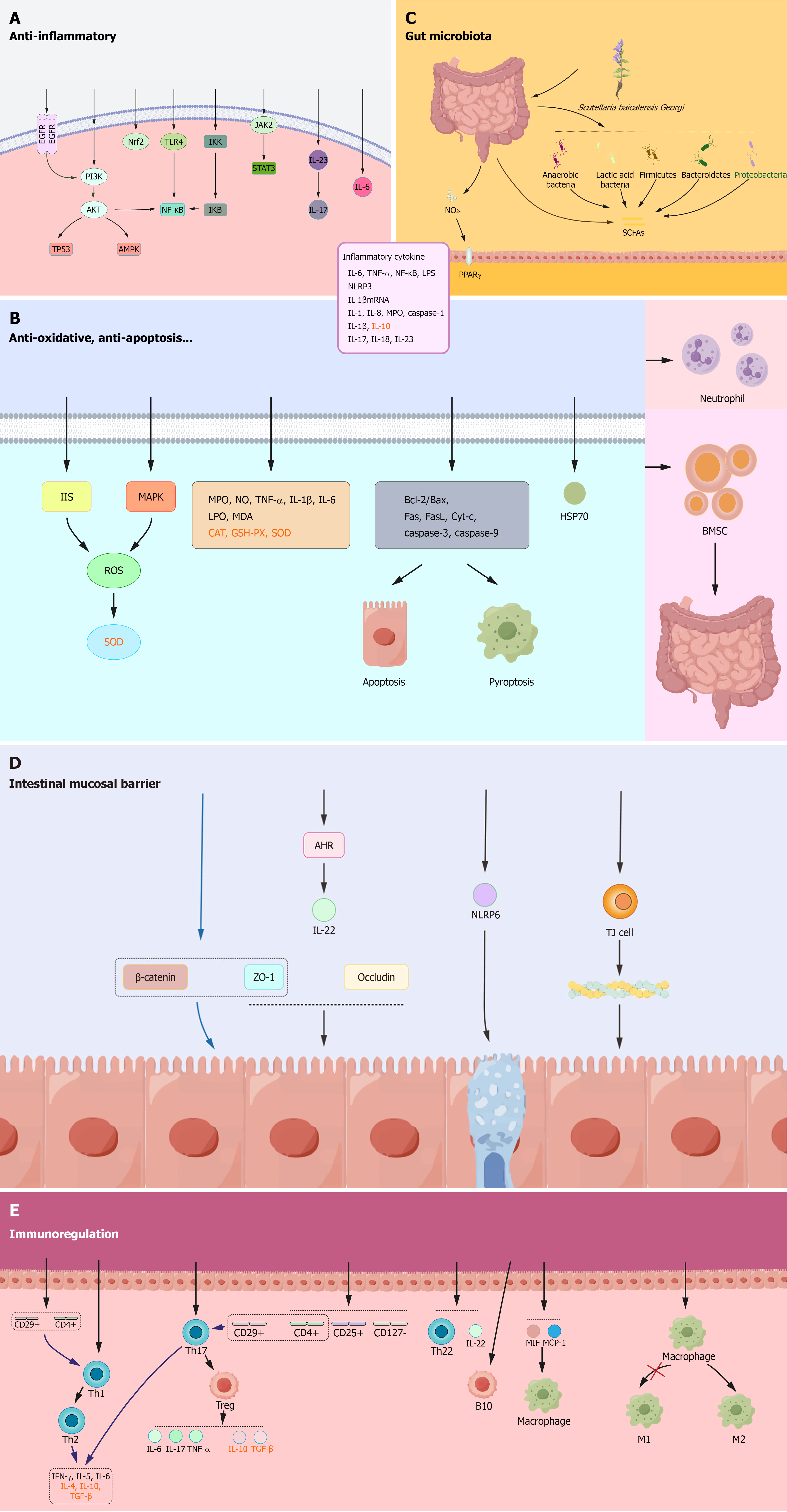
Due to the presence of bioactive components mentioned above, HQ exhibits remarkable therapeutic efficacy in UC treatment and possesses multi-component, multi-target, and multi-pathway characteristics[72]. In this review, we systemically discuss the mechanisms of HQ in treating UC in terms of anti-inflammatory effects, immune cell regulation, protection of the intestinal mucosal barrier, antioxidant activity, anti-apoptotic and pyroptotic effects, and regulation of the gut microbiota (Figure 2).
Inhibition of inflammatory signaling pathways: Baicalein can alleviate colonic inflammation by inhibiting the epidermal growth factor receptor (EGFR)-PI3K/AKT/NF-κB, EGFR/PI3K/AKT/tumor protein p53, and EGFR/PI3K/AKT/MAPK signaling pathways[73]. Wogonin alleviates colonic inflammation by modulating the nuclear factor erythroid-2-related factor 2 and TLR-4/NF-κB signaling pathways, reducing neutrophil infiltration, and exhibiting antioxidant effects in dextran sulphate sodium (DSS)-induced UC mice[74]. Baicalin exerts anti-inflammatory effects by inhibiting various signaling pathways, such as the TLR4/NF-κB p65 signaling pathway[75], the IκB kinase/inhibitor of NF-κB/NF-κB pathway[76], and the PI3K/AKT signaling pathway[77,78]. Scutellarin alleviates inflammatory responses and mitigates colonic inflammatory damage in UC mice by inhibiting activation of the PI3K/AKT/NF-κB signaling pathway[79]. Scutellaria baicalensis polysaccharides improve intestinal mucosal damage and reduce inflammatory cell infiltration in UC mice by suppressing the Janus kinase 2/signal transducer and activator of transcription 3 pathway and the IL-23/IL-17 axis[80].
Regulation of pro-inflammatory/anti-inflammatory cytokines: Baicalein reduces the levels of IL-6, TNF-α, and NF-κB in colonic tissues and serum lipopolysaccharide levels in DSS-induced UC mice[81]. Wogonin reduces intestinal inflammation by inhibiting the formation and activation of the NOD-like receptor protein 3 inflammasome[82]. Baicalin downregulates the levels of IL-1, IL-8, and TNF-α in colonic tissues of trinitrobenzene sulfonic acid (TNBS)-induced UC rats[83] and reduces the expression of myeloperoxidase (MPO) and caspase-1 in DSS-induced mice[84]. Wogonoside downregulates the levels of pro-inflammatory cytokines IL-1β and TNF-α and upregulates the levels of anti-inflammatory cytokine IL-10 in colonic tissues of UC rats[85]. Scutellaria baicalensis polysaccharides significantly downregulate the levels of MPO, TNF-α, IL-6, IL-1β, IL-17, IL-18, and IL-23 in serum and colon tissues of UC mice[80,86]. SP2-1, a polysaccharide isolated from HQ, downregulates the expression of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) in the colon and serum of DSS-induced UC mice, thereby ameliorating UC symptoms[87].
Modulation of T lymphocytes: Baicalein can modulate the Th1/Th2 balance in TNBS-induced UC rats[88]. Baicalein also regulates the Th17/Treg balance by activating the aryl hydrocarbon receptor, downregulating the expression of pro-inflammatory cytokines (such as IL-17, IL-6, and TNF-α), and upregulating the expression of anti-inflammatory cytokines (such as IL-10 and transforming growth factor-β) in DSS-induced UC mice, thereby maintaining intestinal immune homeostasis to treat UC[89]. Wogonin modulates the immune response by upregulating the populations of CD4+, CD44+, and CD8+, CD44+ cells and downregulating the populations of CD4+, CD25+, CD127-, and CD4+, CD25+, Foxp3+ cells in the colon and spleen of DSS-induced UC mice[90]. Baicalin modulates the Th17/Treg balance in TNBS-induced UC rats, ameliorating intestinal inflammation[91]. Baicalin also reduces the percentage of Th22 cells and IL-22 expression in DSS-induced UC mice[92]. Interestingly, baicalin exhibits similar immune regulatory effects in UC patients, and it suppresses inflammatory responses by promoting CD4+, CD29+ T cell proliferation, modulating the Th1/Th2 and Th17/Treg balance, reducing the levels of interferon-γ, IL-5, IL-6, and elevating the levels of IL-4, IL-10, and transforming growth factor-β[93-96].
Regulation of B lymphocytes: Although T cells have traditionally dominated models of UC pathogenesis, increasing evidence shows that B cells play multifaceted roles in the pathogenesis of UC. Single-cell and bulk studies of mucosal tissue have found expansion of naive B cells and IgG+ plasma cells with curtailed diversity and maturation in UC vs healthy controls[97]. The proportions of CD19+ CD5+ B cells (B1 cells) and CD19+ CD5+ CD1d+ B cells (B10 cells) are reduced in patients with UC compared with healthy controls. Notably, baicalin has been shown to upregulate B10 cells, suggesting a potential mechanism for its immunomodulatory effects[98].
Modulation of macrophages: Baicalin regulates the function of macrophages by reducing the expression of macrophage migration inhibitory factor and the level of macrophage chemotactic protein-1 in TNBS-induced UC rats[99]. Baicalin also alleviates colonic inflammation by promoting macrophage polarization to the M2 phenotype[100].
Baicalein may enhance the intestinal barrier function by promoting the aryl hydrocarbon receptor/IL-22 pathway, increasing the expression of two TJ proteins [zonula occludens-1 (ZO-1) and occludin], reducing intestinal permeability, and restoring TJs[101]. Wogonin increases the expression of ZO-1 and occludin and suppresses the expression of pro-inflammatory cytokines by inhibiting the TLR4/myeloid differentiation factor-88/transforming growth factor beta-activated kinase 1 pathway[82,102]. Baicalin may protect the intestinal mucosal barrier by promoting ZO-1 and β-catenin expression, thereby alleviating TNBS-induced colonic inflammation in UC rats[91]. NOD-like receptor family pyrin domain containing 6 (NLRP6) inflammasomes are highly expressed in intestinal epithelial tissues and play a crucial role in regulating intestinal epithelial cells. In DSS-induced UC mice, baicalin increases NLRP6 levels, upregulates TJ proteins, and increases both goblet cell numbers and intestinal mucosal thickness, suggesting that NLRP6 inflammasome activation is a potential mechanism by which baicalin protects goblet cells[103]. Wogonoside promotes TJ protein expression in the intestine, inhibits F-actin rearrangement within the cytoskeleton, and stabilizes the cytoskeletal structure to protect the intestinal mucosal barrier, and this mechanism may be related to the regulation of the myosin light chain kinase/phosphorylated myosin light chain 2 signaling pathway[104]. SP2-1 can upregulate the expression of TJ proteins in the intestine[87].
HQ extract increases the abundance of Lactobacillus, Firmicutes and Bacteroidetes, reduces the abundance of Proteobacteria, and promotes the production of short-chain fatty acids, thereby ameliorating colonic inflammation[105,106]. Wogonin significantly reduces intestinal nitrite levels, decreases the abundance of Enterobacteriaceae, and activates the peroxisome proliferator-activated receptor γ signaling pathway in intestinal epithelial cells in UC mice, thereby alleviating intestinal inflammation[107]. Baicalin promotes the production of short-chain fatty acids and reduces the Firmicutes/Bacteroidetes ratio[91,108], thereby regulating gut microbiota to mitigate intestinal barrier damage. Combining baicalin with berberine hydrochloride enhances gut microbial diversity, potentially through regulating DNA synthesis, replication, and repair in the gut microbiota[109]. SP2-1 ameliorates UC by increasing the abundance of beneficial bacteria, such as Firmicutes, Bifidobacteria, Lactobacillus, and Roseburia, and suppressing harmful bacteria, such as Bacteroides, Proteus, and Staphylococcus in mice[87].
Oxidative stress indicates an imbalance between oxidative and antioxidant processes, characterized by excessive pro
Baicalin significantly reduces the B-cell lymphoma 2/Bcl-2 associated X protein ratio and the levels of Fas, Fas ligand, cytochrome C, caspase-3, and caspase-9 in UC rats, thereby inhibiting colonic epithelial cell apoptosis[76,113], a mecha
Bone marrow-derived mesenchymal stem cells are the most widely utilized stem cells in cell therapy and tissue repair, and one of the advantages is their ability to promote the secretion of anti-inflammatory cytokines[117]. Baicalein enhances the homing of bone marrow-derived mesenchymal stem cells to colonic tissues and improves the restoration of the intestinal mucosal barrier function in UC rats[100,118]. Baicalein also inhibits the expression of the neonatal Fc receptor through the NF-κB signaling pathway, which mitigates pathogenic autoantibody responses and reduces UC recurrence[119]. Furthermore, studies indicate that baicalein elevates heat shock protein 70 expression in the colonic mucosa of TNBS-induced UC rats[120]. Heat shock protein 70, an intestinal protective protein, participates in immune regulation by modulating the release of pro-inflammatory cytokines and acts as a molecular chaperone in colonic epithelial cell repair. Baicalin can downregulate neutrophil infiltration in DSS-induced UC mice, thereby alleviating intestinal inflammation[114]. HQ extracts (with baicalin content verified at 90%-95% by high performance liquid chromatography analysis) have been shown to significantly reduce inflammation and attenuate fibrosis in intestinal epithelial cells and colonic myofibroblasts[121]. One study demonstrated that the anti-inflammatory effects of baicalin are associated with the regulation of autophagic flux[122].
HQ has been widely used as the primary Chinese medicinal herb for treating intestinal disorders, diarrhoea, and dysentery throughout history. In TCM, physicians frequently combine it with other herbs to formulate prescriptions, such as Huangqin decoction, Gegen Qinlian decoction, and Qinlian Lizhong decoction. Extensive clinical studies have confirmed that HQ-containing compound preparations, whether used alone or in combination with Western medicines, can effectively alleviate clinical symptoms, protect the intestinal mucosal barrier, and enhance quality of life in UC patients, with sustained efficacy, reduced recurrence, and minimal adverse drug reactions[11,123-167]. Recent clinical studies on HQ-based formula for UC treatment are summarized in Tables 2, 3 and 4.
| Treatments | Dosage of Huangqin | Number of patients in control/ | Results (overall response rate), % | Adverse events/number of patients | Ref. | ||
| Control groups | Intervention group | ||||||
| Huangqin decoction | Control group: Oral administration of sulfasalazine 3 g/day. Intervention group: Oral Huangqin decoction (huangqin 20 g, baishao 15 g, gancao 15 g, dazao 30 g). Treatment time: 2 months | 20 g | 32/32 | 78.13 | 81.25 | Not reported | [123] |
| Control group: Oral sulfasalazine 3 g/day. Intervention group: Oral Huangqin decoction (huangqin 20 g, baishao 15 g, dazao 30 g, gancao 15 g). Treatment time: 2 months | 20 g | 32/31 | 87.50 | 90.32 | Not reported | [124] | |
| Control group: Oral sulfasalazine 9 tablets/day (dose reduced to 4.5 tablets/day after 2 weeks). Intervention group: Oral modified Huangqin decoction (huangqin 8 g, baishao 6 g, zhigancao 4 g, dazao 8 pieces, adjusted according to symptoms). Treatment time: 1 month | 8 g | 68/68 | 41.18 | 97.06 | Control group: Leukopenia/3, headache and nausea/3, arthritis/1. Intervention group: 0 | [125] | |
| Control group: Oral sulfasalazine 4 g/d. Intervention group: Oral Huangqin decoction granules (huangqin 20 g, baishao 10 g, gancao 6 g, dazao 10 g). Treatment time: 2 months | 20 g | 68/68 | 70 | 86.7 | Not reported | [126] | |
| Control group: Antibiotics, corticosteroids, immunosuppressants, etc. Intervention group: Oral Huangqin decoction (huangqin 15 g, huanglian 10 g, zhizi 10 g, huangbo 10 g). Treatment time: 30 days | 15 g | 50/50 | 68.00 | 90.00 | Not reported | [127] | |
| Group 1: Oral administration of 4 g/day sulfasalazine + 9 tablets/day berberine. Group 2: Retention enema with Chinese patent medicine named Jiechangning. Group 3: Oral modified Huangqin decoction (huangqin 12 g, shaoyao 9 g, zhigancao 6 g, dazao 10 pieces, adjusted according to symptoms). Group 4: Groups 2 plus 3 regimens. Treatment time: 2 months | 12 g | 20/20/20/20 | Group 1: 65. Group 2: 75. Group 4: 95 | 70 | Not reported | [128] | |
| Huangqin decoction plus western medicine | Control group: Oral mesalazine 4 g/day + peifeikang capsules 1.26 g/day. Intervention group: Oral Peifeikang capsules 1.26 g/day + Huangqin decoction (huangqin 20 g, baishao 15 g, gancao 15 g, dazao 15 g, adjusted according to symptoms). Treatment time: 2 months | 20 g | 35/35 | 65.7 | 85.7 | Control group: Mild nausea and vomiting/2. Intervention group: 0 | [129] |
| Control group: Oral sulfasalazine 4 g/day. Intervention group: Control group treatment + Huangqin decoction granules orally (dazao 10 g, huangqin 2 0 g, gancao 6 g, baishao 10 g). Treatment time: Not stated | 20 g | 56/56 | 69.6 | 92.9 | Control group: Arthritis/1, leukopenia/3, headache and nausea/3. Intervention group: 0 | [130] | |
| Control group: Oral mesalazine enteric-coated tablets 3 g/day. Intervention group: Control group treatment + Huangqin decoction granules orally (huangqin 20 g, baishao 10 g, gancao 6 g, dazao 10 g). Treatment time: 2 months | 20 g | 34/34 | 76.5 | 91.2 | Not reported | [131] | |
| Control group: Oral administration of 9 g/day sulfasalazine enteric-coated tablets. Intervention group: Control group treatment + Huangqin decoction orally (huangqin 30 g, baishao 20 g, gancao 20 g, dazao 20 g). Treatment time: 1 month | 30 g | 37/37 | 60.53 | 83.78 | Control group: Nausea/4, fatigue/3, dizziness/1. Intervention group: Nausea/1, fatigue/1 | [11] | |
| Control group: Oral mesalazine granules 3 g/day. Intervention group: Control group treatment + Huangqin decoction granules orally (huangqin 20 g, baishao 10 g, gancao 6 g, dazao 10 g). Treatment time: 2 months | 20 g | 63/63 | 80.95 | 96.83 | Not reported | [132] | |
| Control group: Oral mesalazine 3 g/day. Intervention group: Control group treatment + Huangqin decoction orally (huangqin 25 g, baishao 20 g, dazao 30 g, gancao 12 g, adjusted according to symptoms). Treatment time: 3 months | 25 g | 39/39 | 58.97 | 82.05 | Control group: Nausea and vomiting/2, fatigue/1, dizziness/3, mild skin itching/2. Intervention group: Nausea and vomiting/1, dizziness/1 | [133] | |
| Control group: Mesalazine sustained-release granules 4 g/day orally. Intervention group: Control group treatment plus modified Huangqin decoction administered orally (huangqin 20 g, baishao 15 g, gancao 15 g, dazao 15 g, adjusted according to symptoms). Treatment time: 2 months | 20 g | 41/41 | 68.29 | 90.24 | Not reported | [134] | |
| Control group: Oral mesalazine granules 4 g/day. Intervention group: Control group treatment + modified Huangqin decoction orally (huangqin 20 g, baishao 15 g, gancao 15 g, dazao 15 g, adjusted according to symptoms). Treatment time: 2 months | 20 g | 58/58 | 77.59 | 91.38 | Not reported | [135] | |
| Control group: Oral mesalazine enteric-coated tablets 1.5 g/day. Intervention group: Control group treatment + Huangqin decoction enema (huangqin 9 g, shaoyao 6 g, dazao 12 g, zhigancao 6 g). Treatment time: 30 days | 9 g | 40/40 | 72.50 | 95.00 | Control group: Nausea and vomiting/2, dizziness/1, rash/2. Intervention group: Dizziness/1, rash/1 | [136] | |
| Control group: Oral mesalazine enteric-coated tablets 4 g/day. Intervention group: Control group treatment + Huangqin decoction orally (huangqin 12 g, baishao 15 g, baizhu 15 g, yiyiren 15 g, mudanpi 10 g, diyu 10 g, qinpi 10 g, baiji 6 g, zhigancao 6 g). Treatment time: 2 months | 12 g | 40/40 | 75.00 | 92.50 | Not reported | [137] | |
| Control group: Bifidobacterium quadruple live bacteria tablets 4.5 g + mesalazine enteric-coated tablets 4 g/day orally. Intervention group: Control group treatment + Huangqin decoction enema (dazao 12 pieces, huangqin 9 g, zhigancao 6 g, shaoyao 6 g). Treatment time: 1 month | 9 g | 47/48 | 76.60 | 91.67 | Control group: Nausea/1, vomiting/1, abdominal distension/1. Intervention group: Nausea/2, abdominal distension/2 | [138] | |
| Treatments | Dosage of Huangqin | Number of patients in control/ | Results (overall response rate), % | Adverse events | Ref. | ||
| Control group | Intervention group | ||||||
| Gegen Qinlian decoction | Control group: Oral administration of 1.5 g/day olsarazine sodium capsules. Intervention group: Modified Gegen Qinlian decoction, oral administration (gegen 15 g, huangqin 15 g, huanglian 15 g, zhigancao 15 g, pugongying 15 g, baijiangcao 30 g, huaihua 15 g, baiji 15 g, wubeizi 30 g, gubaipi 60 g, with modifications according to symptoms). Treatment time: 30 days | 15 g | 28/32 | 67.9 | 90.6 | Not reported | [139] |
| Control group received enemas combining the following medications: 150 mL saline solution, 0.6 g Xileisan powder, 3.0 g sulfasalazine, 5 mL lidocaine hydrochloride injection, and 0.2 g metronidazole tablets, mixed thoroughly before administration. Intervention group received an additional modified Gegen Qinlian decoction enema (huangqin 30 g, huanglian 30 g, huangbo 30 g, gegen 30 g, yiyiren 30 g, baihuasheshecao 30 g, baijiangcao 20 g, guangmuxiang 20 g, chishao 20 g). Both groups also received oral herbal decoction. Treatment time: 6 weeks | 30 g | 29/36 | 55.17 | 91.67 | Control group: Leukopenia/1, mild liver function abnormalities/2. Intervention group: Mild liver function abnormality/1 (mild liver enzymes elevated without a detailed statement) | [140] | |
| Control group: Oral sulfasalazine 3 g/day. Intervention group: Gegen Qinlian decoction with pugongying enema (pugongying 45 g, gegen 20 g, huangqin 20 g, huanglian 10 g). Treatment time: 45 days | 20 g | 53/60 | 75.5 | 90 | Not reported | [141] | |
| Control group: Oral sulfasalazine tablets 4 g/day + metronidazole 0.4 g enema. Intervention group: Modified Gegen Qinlian decoction orally (gegen 20 g, huangqin 12 g, huanglian 12 g, baitouweng 12 g, fuling 10 g, cheqianzi 10 g, dangshen 10 g, muxiang 10 g, jineijin 10 g, shanzha 10 g, wumei 6 g, shiliupi 6 g, and zhigancao 6 g, adjusted according to symptoms). Treatment time: 2 months | 12 g | 89/89 | 80.9 | 92.14 | Control group: Facial flushing with headache/2. Intervention group: 0 | [142] | |
| Control group: Mesalazine suppositories rectally twice daily. Intervention group: Modified Gegen Qinlian decoction enema (gegen 30 g, huanglian 15 g, huangqin 20 g, baijiangcao 15 g, machixian 15 g, xianhecao 15 g, baishao 15 g, huangqi 30 g, dangshen 10 g, zhigancao 5 g). Treatment time: 2 months | 20 g | 40/42 | 80.0 | 95.2 | Control group: Gastrointestinal reactions (nausea, vomiting, etc.)/3, mild headache/1. Intervention group: 0 | [143] | |
| Control group: Oral administration of 3-4 g/day of sulfasalazine enteric-coated tablets. Intervention group: Modified Gegen Qinlian decoction enema (gegen 20 g, huanglian 9 g, huangqin 10 g, pugongying 30 g, yiyiren 60 g, baiji 20 g, diyu 15 g, huaihua 10 g, gancao 5 g, adjusted according to symptoms). Treatment time: 30 days | 10 g | 60/60 | 71.7 | 86.7 | Not reported | [144] | |
| Gegen Qinlian decoction plus western medicine | Control group: 0.5% metronidazole 100 mL + sulfasalazine 2.5 g enema. Intervention group: Sulfasalazine 2.5 g + modified Gegen Qinlian decoction enema (gegen 20 g, huangqin 25 g, huanglian 25 g, baiji 30 g, baizhu 18 g, baishao 18 g). Treatment time: 30 days | 25 g | 23/23 | 60.9 | 91.3 | Control group: Gastrointestinal discomfort including nausea, vomiting, loss of appetite, and abdominal distension/7, mild dizziness/1. Intervention group: 0 | [145] |
| Control group: Oral sulfasalazine 1 g/day + sodium succinate hydrocortisone 100 mg enema. Intervention group: Control group therapy + modified Gegen Qinlian decoction orally (gegen 30 g, huangqin 15 g, dangshen 12 g, baizhu 12 g, fuling 30 g, yiyiren 30 g, muxiang 9 g, huoxiang 9 g, huanglian 12 g, dongguaren 30 g, gancao 6 g; adjusted according to symptoms). Treatment time: 1-2 weeks | 15 g | 45/45 | 76.56 | 91.11 | Not reported | [146] | |
| Control group: 3 capsules/d of Baluosulindipine sodium capsules via enema + 4 g/day of sulfasalazine enteric-coated tablets orally. Intervention group: Sulfasalazine enteric-coated tablets 4 g/day orally + Gegen Qinlian decoction enema (gegen 20 g, huangqin 25 g, huanglian 25 g, baiji 30 g, baizhu 18 g, baishao 18 g, danggui 15 g). Treatment time: 1 month | 25 g | 30/32 | 73.33 | 93.75 | Not reported | [147] | |
| Control group: Oral mesalazine 1.5 g/day. Intervention group: Control group treatment + oral administration of Gegen Qinlian decoction (gegen 15 g, huangqin 9 g, huanglian 9 g, gancao 6 g). Treatment time: Control group, more than 2 weeks. Intervention group: 21 days | 9 g | 45/45 | 84.4 | 97.7 | Not reported | [148] | |
| Control group: Bacillus subtilis dual-strain live bacteria capsules 0.75 g/day orally. Intervention group: Control group treatment + Gegen Qinlian decoction enema (baishao 18 g, baizhu 18 g, baiji 30 g, huanglian 25 g, huangqin 25 g, gegen 20 g). Treatment time: 30 days | 25 g | 39/40 | 76.92 | 92.50 | Control group: Gastrointestinal discomfort/2. Intervention group: Mild dizziness/1 | [149] | |
| Control group: Oral sulfasalazine 4 g/day. Intervention group: Control group treatment + oral administration of Gegen Qinlian decoction (gegen 30 g, huanglian 12 g, huangqin 15 g, fuling 30 g, baizhu 12 g, muxiang 9 g, dongguaren 30 g, dangshen 12 g, huoxiang 9 g, yiyiren 30 g, gancao 6 g, adjusted according to symptoms). Treatment time: 1 month | 15 g | 30/30 | 76.67 | 93.33 | Control group: Rash/1, leukopenia/1, elevated transaminases/1; intervention group: Fatigue/1, rash/1 | [150] | |
| Control group: Oral administration of 4 g/day sulfasalazine enteric-coated tablets. Intervention group: Control group therapy + Gegen Qinlian decoction enema (huanglian 23 g, baizhu 20 g, baishaot 15 g, huangqin 24 g, gegen 17 g, danggui 13 g, baiji 29 g). Treatment time: 1 month | 24 g | 48/48 | 83.3 | 95.9 | Not reported | [151] | |
| Control group: Bacillus subtilis and Bacillus licheniformis dual-strain live bacteria capsules 1.5 g/day orally. Intervention group: Control group treatment + Gegen Qinlian decoction enema (gancao 10 g, huangqin 15 g, huanglian 10 g, gegen 30 g). Treatment time: 1 month | 15 g | 16/24 | 75.0 | 91.7 | Not reported | [152] | |
| Control group: Oral mesalazine enteric-coated tablets 1.5 g/day + hydrocortisone succinate sodium 100 mg enema twice daily. Intervention group: Control group treatment + modified Gegen Qinlian decoction orally (gegen 15 g, baitouweng 15 g, dangshen 15 g, baizhu 15 g, fuling 12 g, yiyiren 12 g, huanglian 9 g, huangqin 9 g, huangbo 9 g, shanyao 12 g, danggui 12 g, muxiang 9 g, yanhusuo 9 g, baiji 9 g, sanqi 9 g, gancao 6 g). Treatment time: 1 month | 9 g | 41/41 | 80.49 | 95.12 | Control group: Dizziness and headache/3, nausea and vomiting/3, abdominal distension/1, generalised fatigue/2; intervention group: Nausea and vomiting/1, abdominal distension/1 | [153] | |
| Control group: Mesalazine enteric-coated tablets 3 g/day orally. Intervention group: Control group treatment + modified Gegen Qinlian decoction administered orally (huanglian 3 g, huangqin 10 g, gegen 15 g, machixian 15 g, yiyiren 15 g, shaoyao 10 g, baijiangcao 15 g, hongteng 15 g, fried muxiang 10 g, zhigancao10 g, adjusted according to symptoms). Treatment time: 3 months | 10 g | 30/30 | 70.00 | 93.33 | Control group: 0. Intervention group: Gastric discomfort/1 | [154] | |
| Control group: Oral mesalazine enteric-coated tablets 3 g/day. Intervention group: Control group treatment + modified Gegen Qinlian decoction orally (gegen 15 g, huangqin 15 g, huanglian 5 g, gancao 6 g, zhike 15 g, baizhu 15 g, fuling 10 g). Treatment time: 2 months | 15 g | 40/40 | 72.5 | 92.5 | Not reported | [155] | |
| Control group: Oral mesalazine enteric-coated tablets 3 g/day. Intervention group: Control group treatment + modified Gegen Qinlian decoction orally (yiyiren 30 g, machixian 20 g, chishizhi 20 g, fuling 20 g, gegen, baizhu 15 g, dangshen 15 g, baishao 15 g, muxiang 10 g, chenpi 10 g, huanglian 9 g, huangqin 9 g, gancao 5 g). Treatment time: 1 month | 9 g | 30/30 | 80.00 | 93.33 | Not reported | [156] | |
| Control group: Oral mesalazine enteric-coated tablets 1.5 g/day + hydrocortisone succinate sodium 100 mL enema twice daily. Intervention group: Control group treatment + modified Gegen Qinlian decoction orally (gegen 15 g, baitouweng 15 g, dangshen 15 g, fried baizhu 15 g, yiyiren 12 g, danggui 12 g, shanyao 12 g, fuling 12 g, huanglian 9 g, huangbo 9 g, baiji 9 g, yanhusuo 9 g, sanqi 9 g, muxiang 9 g, huangqin 9 g, gancao 6 g). Treatment time: 1 month | 9 g | 32/32 | 84.38 | 96.88 | Not reported | [157] | |
| Control group: Oral mesalazine sustained-release granules 4 g/day. Intervention group: Control group treatment + modified Gegen Qinlian decoction orally (gegen 15 g, baitouweng 15 g, huangqin 9 g, huanglian 9 g, diyu 9 g, huaihua 9 g, gancao 6 g). Treatment time: 28 days | 9 g | 31/31 | 80.65 | 96.77 | Control group: Intestinal obstruction/3, acute intestinal perforation/1, major intestinal hemorrhage/2. Intervention group: Intestinal obstruction/1 | [158] | |
| Control group: Oral mesalazine enteric-coated tablets 1 g/day + sodium hydrocortisone succinate 50 mg enema twice daily. Intervention group: Control group therapy + modified Gegen Qinlian decoction orally (gegen 5 g, baizhu 5 g, dangshen 5 g, baitouweng 15 g, shanyao 12 g, yiyiren 12 g, fuling 12 g, danggui 12 g, huangbo 9 g, huanglian 9 g, huangqin 9 g, sanqi 9 g, yanhusuo 9 g, muxiang 9 g, baiji 9 g, gancao 6 g). Treatment time: 1 month | 9 g | 51/51 | 76.47 | 92.16 | Not reported | [159] | |
| Control group: Oral mesalazine enteric-coated tablets 3 g/day. Intervention group: Control group treatment + Gegen Qinlian decoction enema (gegen 30 g, huanglian 10 g, gancao 6 g, huangqin 10 g). Treatment time: 2 months | 10 g | 46/48 | 73.91 | 91.67 | Not reported | [160] | |
| Control group: Oral mesalazine enteric-coated tablets 1.5 g/day. Intervention group: Control group treatment + modified Gegen Qinlian decoction orally (gegen 15 g, huanglian 9 g, huangqin 9 g, gancao 6 g, adjusted according to symptoms). Treatment time: 1 month | 9 g | 50/50 | 80.00 | 94.00 | Control group: Abdominal distension/2, indigestion/1, dizziness and headache/2, fatigue/1, rash/1. Intervention group: Abdominal distension/1, indigestion/2, dizziness and headache/1, fatigue/1 | [161] | |
| Control group: Oral mesalazine enteric-coated tablets (4 g/day during acute phase, 1.5 g/day during maintenance phase). Intervention group: Control group treatment + oral administration of Gegen Qinlian decoction (gegen 15 g, huanglian 9 g, huangqin 9 g, gancao 6 g, adjusted according to symptoms). Treatment time: 2 months | 9 g | 39/39 | 82.05 | 97.43 | Not reported | [162] | |
| Treatments | Dosage of Huangqin | Number of patients | Results (overall response rate), % | Adverse events | Ref. | ||
| Control group | Intervention group | ||||||
| Other Huangqin-based formula | Control group: Mesalazine enteric-coated tablets 3 g/day orally. Intervention group: Oral administration of Qinlian Lizhong decoction (huangqin 12 g, huanglian 9 g, baishao 15 g, dangshen 9 g, ganjiang 6 g, baizhu 12 g, xianhecao 15 g, shiliupi 12 g, with adjustments according to symptoms). Treatment time: 2 months | 12 g | 37/38 | 67.6 | 81.6 | Not reported | [163] |
| Control group: Mesalazine enteric-coated tablets 3 g/day orally. Intervention group: Oral administration of Qinlian Lizhong decoction (huangqin 12 g, huanglian 9 g, baishao 15 g, dangshen 9 g, ganjiang 6 g, baizhu 12 g, xianhecao 15 g, shiliupi 12 g, with adjustments according to symptoms). Treatment time: 2 months | 12 g | 57/58 | 70.17 | 84.48 | Control group: Mild nausea and dizziness/1. Intervention group: 0 | [164] | |
| Other Huangqin-based formula plus western medicine | Control group: Oral mesalazine enteric-coated tablets 4 g/day. Intervention group: Control group treatment + compound Qinbai granules 12 g enema (in-house preparation). Treatment time: 2 months | Unknown | 60/60 | 78.33 | 95.00 | Not reported | [165] |
| Control group: Oral mesalazine sustained-release granules 1.5 g/day. Intervention group: Control group treatment + 10 mg Qinbai granules enema (in-house preparation). Treatment time: 3 months | Unknown | 36/37 | 72.2 | 91.9 | Not reported | [166] | |
| Control group: Oral mesalazine sustained-release tablets 0.3 g/day. Intervention group: Control group treatment + Huangqin Shaoyao decoction orally (huangqi 30 g, huangqin 20 g, dangshen 20 g, shaoyao 15 g, jinyinhua 15 g, dazao 15 g, gancao 10 g, baizhu 9 g, danggui 9 g, huanglian 6 g, muxiang 6 g, adjusted according to symptoms). Treatment time: 12 weeks | 20 g | 49/49 | 77.55 | 91.84 | Control group: Nausea and vomiting/1, abdominal distension/2, headache and fatigue/1. Intervention group: Nausea and vomiting/2, abdominal distension/1 | [167] | |
The dosages of HQ used in these clinical studies range from 9 g to 30 g. Reported adverse events (AEs) include nausea, vomiting, retching, dizziness, headache, rash, abdominal distension, mild liver function abnormalities, fatigue, gastric discomfort, intestinal obstruction, and indigestion. Notably, these AEs are also observed in healthy participants, and none of the investigators consider these AEs to be related to HQ. Furthermore, a Phase I, randomized, double-blind, single-dose trial involving 72 healthy participants demonstrates high safety with single oral doses of 100-2800 mg baicalein, with no observed hepatic and renal toxicity[168]. However, Yi et al[169] observed reversible inflammatory changes in liver tissues of rats following 26 weeks of oral administration of 2500 mg/kg/day ethanol extract of HQ, without renal toxicity. Another study reveals renal tubular atrophy and epithelial cell necrosis in male rats administered with baicalin at 200 mg/kg via gavage for 56 days, though no liver damage was observed[170].
Collectively, published studies suggest that HQ may play a beneficial role in the management of UC; however, further experimental and clinical studies are needed to substantiate these findings. HQ exerts therapeutic efficacy in UC through multiple mechanisms, including modulating pro-inflammatory/anti-inflammatory cytokine expression, regulating immune cells, protecting the intestinal barrier, providing antioxidant and anti-apoptotic effects, and modulating the gut microbiota. Its action involves multi-target, multi-pathway, and multi-level characteristics. In clinical practice, HQ formulations, either alone or in combination with western medications, shows favourable outcomes in treating UC, such as reducing adverse reactions to western drugs, enhancing their therapeutic efficacy, and lowering UC recurrence rates. This approach may serve as a reference for future clinical UC management. Although the safety of HQ remains controversial, the absence of reported AEs in clinical studies indicates that HQ at doses between 9-30 g is safe. However, existing research has certain limitations, such as the absence of blinding in all studies, and therapeutic comparisons between oral mesalazine and HQ compound enemas. Regarding mechanistic studies, existing studies predominantly utilize animal models or in vitro approaches, creating a gap with clinical practice. Though HQ contains a wide range of bioactive components, most studies often investigate the mechanisms for individual components rather than the interactions between them. On the other hand, existing clinical trials suffer from small sample sizes, which limits the generalizability and scalability of clinical data. Therefore, future research should focus on analyzing the synergistic mechanisms of multiple components of HQ in large-scale randomized controlled trials, as well as long-term efficacy and safety assessments, to optimize treatment protocols of HQ in UC.
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1653] [Article Influence: 118.1] [Reference Citation Analysis (6)] |
| 2. | Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 934] [Reference Citation Analysis (104)] |
| 3. | Gros B, Kaplan GG. Ulcerative Colitis in Adults: A Review. JAMA. 2023;330:951-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 408] [Reference Citation Analysis (1)] |
| 4. | Zhang S, Zhao L, Shen H, Tang Z, Qin D, Li J, Zhang B, Yang G, Chen M, Wu K, Liu Z, Yang H, Wang H, Zong Y, Chen Y, Xiao S, Cai Q. International clinical practice guideline on the use of traditional Chinese medicine for ulcerative colitis by Board of Specialty Committee of Digestive System Disease of World Federation of Chinese Medicine Societies (2023). Phytother Res. 2024;38:970-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Zhong C, Cheng X, Jia B, Xiong P, Lu J, Zhang P, Liu X, Chen Y. Gancao Xiexin decoction combined with mesalazine in the treatment of ulcerative colitis: A protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e23038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Liu XY, Liu ZX, Tan WW, Zhang WB, Zhang YL, Zheng L, Que RY, Wen HZ, Dai YC. Portulaca Oleracea L. as a Potential Therapeutic Drug Intervention in Ulcerative Colitis: Mechanisms of Action and Clinical Studies. Drug Des Devel Ther. 2024;18:5931-5946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Yan S, Wang P, Wei H, Jia R, Zhen M, Li Q, Xue C, Li J. Treatment of ulcerative colitis with Wu-Mei-Wan by inhibiting intestinal inflammatory response and repairing damaged intestinal mucosa. Phytomedicine. 2022;105:154362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Wang CX, Ge JL, Li F, Zhao KP, Shao SJ, Yang FD, Feng JL. [Effect and Mechanism of Traditional Chinese Medicine in Treatment of Ulcerative Colitis: A Review]. Zhongguo Shiyan Fangjixue Zazhi. 2023;29:270-282. [DOI] [Full Text] |
| 9. | Wu KR, Luo JS, Wu JF, He TM, Tan LX, Li XT. [Meta-analysis of Shenling Baizhu Powder Combined with Aminosalicylic Acid Preparation in the Treat-ment of Ulcerative Colitis]. Zhongguo Yaofang. 2017;28:5119-5122. [DOI] [Full Text] |
| 10. | Niu BB, Ye SF, Chen BY, Zhang M, He DG, Wang X. [Mesalamine combined with Kangfuxin Liquid in the treatment of ulcerative colitis: A meta-analysis]. Zhongchengyao. 2014;36:2275-2279. |
| 11. | Chen YH, Cao QF, Hong QK, Lin SZ, Sun JL. [Effect of Huangqin Decoction Combined with Sulfasalazine on Serum TNF-α and Interleukin of Colitis Gravis Patients]. Zhonghua Zhongyiyao Xuekan. 2017;35:500-503. [DOI] [Full Text] |
| 12. | State Pharmacopoeia Commission of the People's Republic of China. Pharmacopoeia of the People’s Republic of China, Volume I, 2020 Edition. Beijing: China Pharmaceutical Technology Press, 2020. |
| 13. | Qian JX, Meng WW, Zhao JC, Wang YH, Jin Y, Zhan ZL. [Herbal Textual Research on Scutellariae Radix in Famous Classical Formulas]. Zhongguo Shiyan Fangjixue Zazhi. 2023;29:84-93. [DOI] [Full Text] |
| 14. | Zheng XB, Feng YL, Liu HB, Dai SX. [Effect of Huangqin Decoction on CD4^+T Cell and its Co-stimulator Factors for Unlcerative Colitis Rat with Damp-and-heat Syndrome]. Zhongguo Shiyan Fangjixue Zazhi. 2011;17:169-172. [DOI] [Full Text] |
| 15. | Li YL, Liu JJ, Ma PG, Dong RJ, Ge DY, Liu HS, Peng GY, Li JX. [Gegen Qinliantang Regulates MMP-9/p38 MARK Pathway to Repair Intestinal Mucosal Barrier Function in Mice with Ulcerative Colitis]. Zhongguo Shiyan Fangjixue Zazhi. 2021;27:8-15. [DOI] [Full Text] |
| 16. | Wang T, Liu X, Zhang W, Wang J, Wang T, Yue W, Ming L, Cheng J, Sun J. Traditional Chinese medicine treats ulcerative colitis by regulating gut microbiota, signaling pathway and cytokine: Future novel method option for pharmacotherapy. Heliyon. 2024;10:e27530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Wang ZL, Wang S, Kuang Y, Hu ZM, Qiao X, Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol. 2018;56:465-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 267] [Article Influence: 33.4] [Reference Citation Analysis (7)] |
| 18. | Qiao X, Li R, Song W, Miao WJ, Liu J, Chen HB, Guo DA, Ye M. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J Chromatogr A. 2016;1441:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Jiang XH, Liu SM. [Research on Pharmacological Effects and Substance Basis of Scutellaria Baicalensis]. Zhongguo Yaoshi. 2020;23:2004-2010. |
| 20. | Wang YL, Liu W, Yang DB, Qing YJ, Du P. [Research on Spectrum-Effect Relationship of Scutellaria baicalensis Georgi]. Zhonghua Zhongyiyao Xuekan. 2017;35:3110-3113. [DOI] [Full Text] |
| 21. | Liao H, Ye J, Gao L, Liu Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed Pharmacother. 2021;133:110917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 22. | Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 404] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 23. | Bui TT, Piao CH, Song CH, Lee CH, Shin HS, Chai OH. Baicalein, wogonin, and Scutellaria baicalensis ethanol extract alleviate ovalbumin-induced allergic airway inflammation and mast cell-mediated anaphylactic shock by regulation of Th1/Th2 imbalance and histamine release. Anat Cell Biol. 2017;50:124-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Kim DH, Hossain MA, Kang YJ, Jang JY, Lee YJ, Im E, Yoon JH, Kim HS, Chung HY, Kim ND. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int J Oncol. 2013;43:1652-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Wang C, Cui C. Inhibition of Lung Cancer Proliferation by Wogonin is Associated with Activation of Apoptosis and Generation of Reactive Oxygen Species. Balkan Med J. 2019;37:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hong M, Almutairi MM, Li S, Li J. Wogonin inhibits cell cycle progression by activating the glycogen synthase kinase-3 beta in hepatocellular carcinoma. Phytomedicine. 2020;68:153174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Meng X, Ning C, Kang M, Wang X, Yu Z, Hao X, Guo H. Baicalin: Natural Sources, Extraction Techniques, and Therapeutic Applications Against Bacterial Infections. Molecules. 2025;30:3464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wu MH, Huang YQ, Huang ZS, Zhou XH, Yu WQ, Su JW. [In vitro bacteriostatic effect of berberine, emodin, schisandra, and baicalin on multidrug resistant strains of Helicobacter pylori]. Shijie Huaren Xiaohua Zazhi. 2013;21:3247-3251. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Qiu R, Li J, Jiang C, Yu Y, Li D, Xie X, Lei Y, Yao L. Antibacterial activity of baicalein against Aeromonas hydrophila: in vitro and in vivo evaluation. Front Microbiol. 2025;16:1615029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Sun X, Tao JL, Xu SJ, Yuan B. [The molecular mechanism of treating COVID-19 with Huashi Baidu formula based on network pharmacology]. Zhongyaocai. 2020;43:2047-2052. [DOI] [Full Text] |
| 31. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2710] [Article Influence: 301.1] [Reference Citation Analysis (2)] |
| 32. | Ma XX, Wen YT, Yin XL, Zhang BH, Tang XD. [To Explore the Mechanism of Intestinal Mucosal Immunity and Ulcerative Colitis in Chinese Medicine from Perspective of “Spleen as the Defense”]. Shijie Kexue Jishu-Zhongyiyao Xiandaihua. 2024;26:640-645. |
| 33. | Jiang Y, Zhao QF, Wang S, Luo LH, Xu PZ. [Analysis of relationship between intestinal flora imbalance and ulcerative colitis based on 16S rRNA sequences]. Shijie Huaren Xiaohua Zazhi. 2017;25:3191-3202. [RCA] [DOI] [Full Text] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn's disease. Gastroenterol Res Pract. 2013;2013:431231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 35. | Kałużna A, Olczyk P, Komosińska-Vassev K. The Role of Innate and Adaptive Immune Cells in the Pathogenesis and Development of the Inflammatory Response in Ulcerative Colitis. J Clin Med. 2022;11:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 36. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 706] [Article Influence: 100.9] [Reference Citation Analysis (2)] |
| 37. | Cronkite DA, Strutt TM. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J Immunol Res. 2018;2018:1467538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 38. | Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016;311:G59-G73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Zhu YZ, Dai J, Yao XY, Dong GJ, Zhang H, Yan FL, Li CX, Li XH, Xiong HB, Si CP. [IL-16 aggravates dextran sulfate sodium (DSS)-induced mouse inflammatory bowel disease by promoting M1 polarization of macrophages]. Xibao Yu Fenzi Mianyixue Zazhi. 2018;34:695-701. [DOI] [Full Text] |
| 40. | Pai RK, Hartman DJ, Rivers CR, Regueiro M, Schwartz M, Binion DG, Pai RK. Complete Resolution of Mucosal Neutrophils Associates With Improved Long-Term Clinical Outcomes of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:2510-2517.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1980] [Article Influence: 132.0] [Reference Citation Analysis (2)] |
| 42. | Dong Z, Du L, Xu X, Yang Y, Wang H, Qu A, Qu X, Wang C. Aberrant expression of circulating Th17, Th1 and Tc1 cells in patients with active and inactive ulcerative colitis. Int J Mol Med. 2013;31:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Chang YY, Ouyang Q. [Expression and significance of mucosal beta-defensin-2, TNFalpha and IL-1beta in ulcerative colitis]. Zhonghua Nei Ke Za Zhi. 2008;47:11-14. [PubMed] |
| 44. | Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński T, Zwoździak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz). 2009;57:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Lv W, Han S, Li K, Yan A, Wang W, Lu W, Han J, Zhang C. Baicalein ameliorates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and regulating gut microbiota. Front Pharmacol. 2025;16:1564783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Zhou ZJ, Dong JY, Qiu Y, Zhang GL, Wei K, He LH, Sun YN, Jiang HZ, Zhang SS, Guo XR, Wang JY, Chen DP. Sulforaphane decreases oxidative stress and inhibits NLRP3 inflammasome activation in a mouse model of ulcerative colitis. Biomed Pharmacother. 2024;175:116706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Liang XJ, Ma ZM, Zeng YJ. [The clinical significance of interleukin 4 &interleukin 17's blood plasma level in patients with ulcerative colitis]. Youjiang Yixue. 2005;6-8. |
| 48. | Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D'Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 862] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 49. | Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 932] [Article Influence: 58.3] [Reference Citation Analysis (1)] |
| 50. | Laudisi F, Stolfi C, Monteleone G. Impact of Food Additives on Gut Homeostasis. Nutrients. 2019;11:2334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1643] [Cited by in RCA: 1729] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 52. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1983] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 53. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (116)] |
| 54. | Sun M, Liu Y, Song Y, Gao Y, Zhao F, Luo Y, Qian F, Mu G, Tuo Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020;11:5205-5222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Zhang F, Li Y, Wang X, Wang S, Bi D. The Impact of Lactobacillus plantarum on the Gut Microbiota of Mice with DSS-Induced Colitis. Biomed Res Int. 2019;2019:3921315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Liu L, Liang L, Liang H, Wang M, Lu B, Xue M, Deng J, Chen Y. Fusobacterium nucleatum Aggravates the Progression of Colitis by Regulating M1 Macrophage Polarization via AKT2 Pathway. Front Immunol. 2019;10:1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 57. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8109] [Article Influence: 506.8] [Reference Citation Analysis (4)] |
| 58. | Yu JJ, Li H, Hu QY, Wei S, Wang Q, Fang SY, Wu QM. [Research on Gut Microbiota Diversity in Patients with Ulcerative Colitis by High-throughput Sequencing]. Huazhong Keji Daxue Xuebao (Yixueban). 2018;47:460-465. |
| 59. | Sun Y, Ding YQ. [Changes of intestinal flora and pathology in ulcerative colitis]. Xiandai Xiaohua Ji Jieru Zhenliao. 2009;14:26-28. |
| 60. | Singh N, Bernstein CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 61. | Gaidos J, Kang JD, Kakiyama G, Pandak WMJ, Hylemon PB. Pilot Study of Gut Dysbiosis and Bile Acid Dysmetabolism in Active Ulcerative Colitis. Am J Gastroenterol. 2019;114:S438. [DOI] [Full Text] |
| 62. | Keshteli AH, Madsen KL, Dieleman LA. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients. 2019;11:1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 63. | Chen YH, Bai J, Wu D, Yu SF, Qiang XL, Bai H, Wang HN, Peng ZW. Association between fecal microbiota and generalized anxiety disorder: Severity and early treatment response. J Affect Disord. 2019;259:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 64. | Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 788] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 65. | Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal Barrier in Human Health and Disease. Int J Environ Res Public Health. 2021;18:12836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (1)] |
| 66. | Tan Y, Zheng CQ. [Expression and clinical significance of tight junction protein occludin and ZO-1 in ulcerative colitis]. Xiandai Yaowu Yu Linchuang. 2018;33:1803-1808. |
| 67. | Du XD, Luo LF. [Changes in intestinal flora and intestinal mucosal barrier of patients with ulcerative colitis and the effect of probiotics intervention]. Zhongguo Weishengtaixue Zazhi. 2019;31:193-196. [DOI] [Full Text] |
| 68. | Kayal M, Shah S. Ulcerative Colitis: Current and Emerging Treatment Strategies. J Clin Med. 2019;9:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 69. | Min S, Fang Y, Zhang M, Shen H, Zhu L. The potential mechanism of co-administration of Scutellaria baicalensis Georgi and Rubia cordifolia L ameliorating ulcerative colitis: Integration of metabolomics, network pharmacology, and molecular docking. J Pharm Biomed Anal. 2025;263:116948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 70. | Teschke R, Wolff A, Frenzel C, Eickhoff A, Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J Gastroenterol. 2015;21:4466-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 71. | Zhu QF, Liu L. Research Progress of Chinese Medicine in Regulating Relevant Signalling Pathways for the Treatment of Ulcerative Colitis. J Contemp Med Pract. 2024;6:94-98. [DOI] [Full Text] |
| 72. | Dong X, Wang FJ, Ju ZH, Ge HT. [Study on Effect and Mechanism of Total Flavonoids from Stem and Leaf of Scutellaria Baicalensis on Ulcerative Colitis Based on Network Pharmacology and Animal Experiments]. Zhongguo Xiandai Yingyong Yaoxue. 2024;41:1183-1191. [DOI] [Full Text] |
| 73. | Liu A, Zhong M, Han Z, Yan Y, Zhang D, Wang X, Wang M, Zou Y, Zhang J. Characterization of Active Compounds in Sanhuang Shu'ai Decoction for the Management of Ulcerative Colitis: A UHPLC-MS Study. Rapid Commun Mass Spectrom. 2025;39:e9976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 74. | Zhou Y, Dou F, Song H, Liu T. Anti-ulcerative effects of wogonin on ulcerative colitis induced by dextran sulfate sodium via Nrf2/TLR4/NF-κB signaling pathway in BALB/c mice. Environ Toxicol. 2022;37:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Fu YJ, Xu B, Huang SW, Luo X, Deng XL, Luo S, Liu C, Wang Q, Chen JY, Zhou L. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 76. | Shen J, Cheng J, Zhu S, Zhao J, Ye Q, Xu Y, Dong H, Zheng X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int Immunopharmacol. 2019;73:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 77. | Zhu L, Shen H, Gu PQ, Liu YJ, Zhang L, Cheng JF. Baicalin alleviates TNBS-induced colitis by inhibiting PI3K/AKT pathway activation. Exp Ther Med. 2020;20:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 78. | Feng J, Guo C, Zhu Y, Pang L, Yang Z, Zou Y, Zheng X. Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int J Clin Exp Med. 2014;7:4063-4072. [PubMed] |
| 79. | Zhang Z, Hu P. [Effects of Scutellarin on Colonic Inflammatory Injury in Mice with Ulcerative Colitis by Regulating PI3K/Akt/NF-κB Signaling Pathway]. Guangzhou Zhongyiyao Daxue Xuebao. 2025;42:718-725. [DOI] [Full Text] |
| 80. | Ma J, Chen YZ, Tian L. [Scutellaria Baicalensis Polysaccharides Alleviates the Inflammation of DSS-induced UC Model Mice by Regulating JAK 2/STAT 3 Pathway and IL-23/IL-17 Inflammatory Axis]. Zhongshan Daxue Xuebao (Yixue Kexueban). 2023;44:423-429. [DOI] [Full Text] |
| 81. | Cheng NN, Zheng CX, Dai X, Hu Y, Li XZ, Sun C. [Protective effect of baicalein on mice with ulcerative colitis induced by dextran sulfate sodium salt]. Zhongguo Xinyao Zazhi. 2023;32:276-282. |
| 82. | Liu Q, Zuo R, Wang K, Nong FF, Fu YJ, Huang SW, Pan ZF, Zhang Y, Luo X, Deng XL, Zhang XX, Zhou L, Chen Y. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway. Acta Pharmacol Sin. 2020;41:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 83. | Zhu L, Shen H, Gu PQ, Jiang Y, Liu YJ, Zhang L, Cheng JF. [Effect of Baicalin on Expression of NF-κB in Ulcerative Colitis Rats]. Nanjing Zhongyiyao Daxue Xuebao. 2016;32:447-450. [DOI] [Full Text] |
| 84. | Li KY, Xiao JY, Luo WP, Lu WH, He LZ, Pan L, Zhao JZ, Cheng SY, Xiao LM, Wang JW, Wang ZQ. [Mechanism of baicalin modulation of TUG1/PTBP1/NLRP3 molecular network to inhibit macrophage pyroptosis in treatment of ulcerative colitis]. Zhongcaoyao. 2025;56:1667-1681. |
| 85. | Zhang X, Du WZ, Zhao HQ, Zhang S. [Effects of Wogonoside on Pro-inflammatory Factors, Oxidative Stress Markers and Mucosal Repair in Rats with Ulcerative Colitis]. Zhongguo Laonianxue Zazhi. 2022;42:2994-2998. |
| 86. | Cui L, Ning Q, Zhang RT, Liu GG, Zhong RL, Xia Z, Wang J. [Optimization of Extraction Condition for Polysaccharides from Scutellaria Baicalensis Georgi and Study of Its Effect on Mice with Ulcerative Colitis]. Shandong Zhongyi Zazhi. 2020;39:993-1000. [DOI] [Full Text] |
| 87. | Cui L, Guan X, Ding W, Luo Y, Wang W, Bu W, Song J, Tan X, Sun E, Ning Q, Liu G, Jia X, Feng L. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol. 2021;166:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 88. | Kuang Z, Guo HL, Zhang YC, Miao ZW. [Effects of baicalein on protein expression of Th1/2 cells, obestatin and 5-hydroxytryptamine in rats with ulcerative colitis]. Zhongguo Linchuang Yanjiu. 2024;37:895-900. [DOI] [Full Text] |
| 89. | Liu C, Li Y, Chen Y, Huang S, Wang X, Luo S, Su Y, Zhou L, Luo X. Baicalein Restores the Balance of Th17/Treg Cells via Aryl Hydrocarbon Receptor to Attenuate Colitis. Mediators Inflamm. 2020;2020:5918587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 90. | Xiao W, Yin M, Wu K, Lu G, Deng B, Zhang Y, Qian L, Jia X, Ding Y, Gong W. High-dose wogonin exacerbates DSS-induced colitis by up-regulating effector T cell function and inhibiting Treg cell. J Cell Mol Med. 2017;21:286-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Zhu L, Xu LZ, Zhao S, Shen ZF, Shen H, Zhan LB. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl Microbiol Biotechnol. 2020;104:5449-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 92. | Zhao B, Zou Y, Zheng XB, Guo CC, Li WY, Chi HG. [Effect of Baicalin on Th22 and IL-22 in DSS-induced Colitis Mice]. Shijie Kexue Jishu-Zhongyiyao Xiandaihua. 2015;17:1254-1261. |
| 93. | Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. [The correlation between CD4+CD29+T cell subsets and ulcerative colitis and the intervention effect of baicalin]. Shijie Huaren Xiaohua Zazhi. 2014;22:3710-3717. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. [Influence of Baicalin on Expression of Cytokines in Ulcerative Colitis Patients]. Guangzhou Zhongyiyao Daxue Xuebao. 2014;31:864-872. [DOI] [Full Text] |
| 95. | Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. [The baicalin right patients with ulcerative colitis NF-kappa B and phosphorylation of NF-kappa B expression]. Zhongguo Linchuang Yanjiu. 2014;6:14-18. |
| 96. | Yu FY, Huang SG, Zhang HY, Chi HG, Zou Y, Lv RX, Zheng XB. [Changes of Peripheral Blood RORC and Foxp3 Mrna Expression Levels of Ulcerative Colitis Patients and Intervention Effect of Baicalin]. Liaoning Zhongyi Zazhi. 2014;41:225-229. [DOI] [Full Text] |
| 97. | Uzzan M, Martin JC, Mesin L, Livanos AE, Castro-Dopico T, Huang R, Petralia F, Magri G, Kumar S, Zhao Q, Rosenstein AK, Tokuyama M, Sharma K, Ungaro R, Kosoy R, Jha D, Fischer J, Singh H, Keir ME, Ramamoorthi N, O'Gorman WE, Cohen BL, Rahman A, Cossarini F, Seki A, Leyre L, Vaquero ST, Gurunathan S, Grasset EK, Losic B, Dubinsky M, Greenstein AJ, Gottlieb Z, Legnani P, George J, Irizar H, Stojmirovic A, Brodmerkel C, Kasarkis A, Sands BE, Furtado G, Lira SA, Tuong ZK, Ko HM, Cerutti A, Elson CO, Clatworthy MR, Merad M, Suárez-Fariñas M, Argmann C, Hackney JA, Victora GD, Randolph GJ, Kenigsberg E, Colombel JF, Mehandru S. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med. 2022;28:766-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 98. | Feng JS, Wang SQ, Ye Y, Zhou XG, Zheng XB. [Expression of B1 and B10 Cells in Peripheral Blood of Patients with Ulcerative Colitis and Baicalin Effect on its Expression in vitro]. Zhongguo Shiyan Fangjixue Zazhi. 2013;19:245-248. |
| 99. | Dai SX, Zou Y, Feng YL, Liu HB, Zheng XB. Baicalin down-regulates the expression of macrophage migration inhibitory factor (MIF) effectively for rats with ulcerative colitis. Phytother Res. 2012;26:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F, Shi X, Zhu X, Zhang X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int Immunopharmacol. 2016;35:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 101. | Li YY, Wang XJ, Su YL, Wang Q, Huang SW, Pan ZF, Chen YP, Liang JJ, Zhang ML, Xie XQ, Wu ZY, Chen JY, Zhou L, Luo X. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol Sin. 2022;43:1495-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 102. | Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res. 2015;64:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 103. | Li Y, Hu J, Cheng C, Xu F, Au R, Zhu L, Shen H. Baicalin Ameliorates DSS-Induced Colitis by Protecting Goblet Cells through Activating NLRP6 Inflammasomes. Evid Based Complement Alternat Med. 2022;2022:2818136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 104. | Huang S, Fu Y, Xu B, Liu C, Wang Q, Luo S, Nong F, Wang X, Huang S, Chen J, Zhou L, Luo X. Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway. Phytomedicine. 2020;68:153179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 105. | Liu SF, Luo YS, Liu PL, Kang XC, Wang L, Wu RY, Liu DB. [Effects of Scutellaria baicalensis Extracts on Intestinal Flora and Short-chain Fatty Acids]. Zhongguo Shiwu Yu Yingyang. 2021;27:49-53. [DOI] [Full Text] |
| 106. | Huang W, Luo YQ, Yu XY, Dong HJ, Wang X. [Effect of extracts of Pith-nodecayed and Pith-decayed of Scutellariae Radix on intestinal flora and protective mechanism of intestinal mucosal barrier in damp-heat ulcerative colitis rats]. Zhongguo Yaowu Jingjie. 2024;21:408-414. [DOI] [Full Text] |
| 107. | Su Y, Liang J, Zhang M, Zhao M, Xie X, Wang X, Pan Z, Huang S, Yan R, Wang Q, Zhou L, Luo X. Wogonin regulates colonocyte metabolism via PPARγ to inhibit Enterobacteriaceae against dextran sulfate sodium-induced colitis in mice. Phytother Res. 2023;37:872-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 108. | Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li L, Li Z, Peng X, Wei S, Ma X, Zhao Y. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol Res. 2021;165:105444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 109. | Yan Y, Li L, Wu K, Zhang G, Peng L, Liang Y, Wang Z. A Combination of Baicalin and Berberine Hydrochloride Ameliorates Dextran Sulfate Sodium-Induced Colitis by Modulating Colon Gut Microbiota. J Med Food. 2022;25:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 110. | Tian T, Wang Z, Zhang J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid Med Cell Longev. 2017;2017:4535194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 477] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 111. | Wang W, Fu X, Xu J, Lv W, Han S, Wang Y, Xia Y, Han J, Li K, Zhang C. Baicalein from Scutellaria baicalensis mitigates oxidative stress through the IIS pathway in a C. elegans model of ulcerative colitis. Front Pharmacol. 2025;16:1592244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 112. | Chen SR, Tang JT, Li WB. [Baicalin mediates GPX4 to regulate ferroptosis in the treatment of ulcerative colitis]. Jiepou Kexue Jinzhan. 2025;31:305-308. [DOI] [Full Text] |
| 113. | Yao J, Cao X, Zhang R, Li YX, Xu ZL, Zhang DG, Wang LS, Wang JY. Protective Effect of Baicalin Against Experimental Colitis via Suppression of Oxidant Stress and Apoptosis. Pharmacogn Mag. 2016;12:225-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Zhang CL, Zhang S, He WX, Lu JL, Xu YJ, Yang JY, Liu D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017;186:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 115. | Chong J, Chen Z, Ma J, He L, Zhu Y, Lu Z, Qiu Z, Chen C, Chen Y, Jiang F. Mechanistic investigation and the optimal dose based on baicalin in the treatment of ulcerative colitis-A preclinical systematic review and meta-analysis. BMC Gastroenterol. 2025;25:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 116. | Zhang J, Tan B, Wu H, Han T, Fang D, Cai H, Hu B, Kang A. Scutellaria baicalensis Extracts Restrict Intestinal Epithelial Cell Ferroptosis by Regulating Lipid Peroxidation and GPX4/ACSL4 in Colitis. Phytomedicine. 2025;141:156708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 117. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 727] [Article Influence: 103.9] [Reference Citation Analysis (36)] |
| 118. | Chen JG, Zhou L, Wang Y, Zhang K. [Study on the function of Baicalein in promoting the homing of bone marrow mesenchymal stem cells and contributing to the treatment of ulcerative colitis]. Shiyong Yaowu Yu Linchuang. 2018;21:871-875. [DOI] [Full Text] |
| 119. | Hu H, Lu F, Guan X, Jiang X, Wen C, Wang L. Baicalein Ameliorates Experimental Ulcerative Colitis Recurrency by Downregulating Neonatal Fc Receptor via the NF-κB Signaling Pathway. ACS Omega. 2025;10:10701-10712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 120. | Xu WH, Zhang LZ, Li X, Wang JH, Zhao G. [Effects of baicalein on heat shock protein 70 expression in rats with ulcerative colitis]. Zhongguo Linchuang Yaolixue Zazhi. 2020;36:36-38. [DOI] [Full Text] |
| 121. | Laudadio I, Leter B, Palone F, Cucchiara S, Carissimi C, Scafa N, Secci D, Vitali R, Stronati L. Inhibition of intestinal inflammation and fibrosis by Scutellaria Baicalensis georgi and Boswellia serrata in human epithelial cells and fibroblasts. Immun Inflamm Dis. 2024;12:e70036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 122. | Rizzo V, Ferlazzo N, Currò M, Isola G, Matarese M, Bertuccio MP, Caccamo D, Matarese G, Ientile R. Baicalin-Induced Autophagy Preserved LPS-Stimulated Intestinal Cells from Inflammation and Alterations of Paracellular Permeability. Int J Mol Sci. 2021;22:2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 123. | Yu XF, Lv XZ, Dong WB. [Clinical observation of Huangqin decoction in treating Ulcerative Colitis]. Zhongguo Zhongyi Jizheng. 2010;19:1510-1529. [DOI] [Full Text] |
| 124. | Kuang ZQ. [Scutellaria Decoction Treatment of Ulcerative Colitis Random Parallel Control Study]. Shiyong Zhongyi Neike Zazhi. 2014;28:55-57. [DOI] [Full Text] |
| 125. | Chen JW, Zhang GK, Huang YX, Cheng FW, Ma JH. [Clinical Study on Modified Huangqin decoction for Treating Ulcerative Colitis]. Heilongjiang Yiyao. 2015;28:564-566. |
| 126. | Shi HH, Zhao XF, Han R. [Therapeutic Observation of Huangqin Decoction Granules in Treating Damp-Heat Pattern Ulcerative Colitis]. Zhongwen Keji Qikan Shujuku (Yinwenban) Yiyao Weisheng. 2022;260-263. |
| 127. | Huang WL, Huang NC. [Clinical Observation of Compound Huangqin decoction in Treating Damp-Heat Pattern Ulcerative Colitis]. Zhongwen Keji Qikan Shujuku (Yinwenban) Yiyao Weisheng. 2024;064-067. |
| 128. | Zeng S, Wu Y. [Modified Huangqin decoction Combined with Retention Enema of Colon Ning for Treating Ulcerative Colitis: A Study of 20 Cases]. Hunan Zhongyi Zazhi. 2013;39-40. |
| 129. | Liu YP, Wang ZM, Liu MS, Li QS, Yang CY. [Scutellaria Decoction Combined with Bifico Capsule Intervention Effect on Ulcerative Colitis and Its Influence on Immune Function]. Zhonghua Zhongyiyaoxue Kan. 2015;33:1522-1526. [DOI] [Full Text] |
| 130. | Wu YK. [Treatment of damp-heat pattern Ulcerative Colitis with Huangqin Decoction Granules]. Zhongguo Yiyao Zhinan. 2016;14:187-188. [DOI] [Full Text] |
| 131. | Yang M, Wu D. [Clinical efficacy study of Huangqin Decoction Granules combined with mesalazine for Ulcerative Colitis]. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2016;24:221-223. |
| 132. | Ding HR, Wang BX, Yang Y, Li X, Wang HW. [Effect of Scutellaria Decoction Granules combined with mesalazine in treating ulcerative colitis and its influence on inflammatory factors]. Jianyan Yixue Yu Linchuang. 2018;15:806-809. |
| 133. | Wang HL, Wang XL. [Huangqin Decoction with Modifications as an Adjuvant to Mesalazine in Treating 39 Cases of Ulcerative Colitis]. Xiandai Zhongyiyao. 2019;39:56-58. |
| 134. | Yan L. [Clinical observation of modified Huangqin decoction combined with mesalazine in treating Ulcerative Colitis]. Sichuan Zhongyi. 2019;37:101-103. |
| 135. | Hu J, Guo XT, Zhou DC, Jiang DP, Jin WQ, Li XJ, Yang W. [Effect of Huangqin Decoction Combined with Mesalazine on Inflammatory Factors of Intestinal Mucosa in Patients with Ulcerative Colitis (Active Stage)]. Zhonghua Zhongyiyao Xuekan. 2021;39:123-126. |
| 136. | Wang YT, Li LD, Yu GH, Wu XM, Guo L. [Effect of Huangqin Decoction and Enema Combined Therapy on Ulcerative Colitis]. Zhongguo Weisheng Biaozhun Guanli. 2022;13:143-147. |
| 137. | Xuan L, Wang XH, Hu B, Sun FX. [Compound Huangqin Decoction in the Treatment of Dampness-heat Ulcerative Colitis]. Guangming Zhongyi. 2024;39:281-284. |
| 138. | Wang HY, Zhang HB. [Therapeutic Observation of Huangqin decoction Enema Combined with Probiotics and Mesalazine in the Treatment of Ulcerative Colitis]. Shiyong Zhongyiyao Zazhi. 2024;40:2476-2479. |
| 139. | He JB, Yang XD. [Therapeutic efficacy of modified Gegen Qinlian decoction for damp-heat pattern Ulcerative Colitis in the large intestine]. Sichuan Zhongyi. 2013;31:104-105. |
| 140. | Yuan Y, Chen HW. [Clinical Observation on Puerariae and Scutellariae and Coptidis Decoction in Treatment of 60 Patients with Ulcerative Colitis]. Xiandai Shengwu Yixue Jinzhan. 2007;1336-1337. [DOI] [Full Text] |
| 141. | Xie MJ. [Therapeutic Effect of Retention-enema with Modified Gegen Qinlian Decoction for Damp-heat Ulcerative Colitis]. Guangzhou Zhongyiyao Daxue Xuebao. 2013;30:491-494. [DOI] [Full Text] |
| 142. | Wang J. [Modified Kudzu Root, Gegen Qinlian Decoction for Treating 89 Cases of Damp-Heat Pattern Ulcerative Colitis]. Sichuang Zhongyi. 2012;30:88-89. |
| 143. | Zhang QY, Xiao XB. [Therapeutic Observation of Modified Kudzu Root, Gegen Qinlian Decoction Enema for Damp-Heat Accumulation Pattern of Ulcerative Colitis]. Qiuyi Wenyao. 2012;10:605-606. |
| 144. | Wang ZM, Zhang FF. [Clinical observation of 60 cases of Ulcerative Colitis treated with modified Gegen Qinlian Decoction combined with retention enema]. Jiangsu Zhongyiyao. 2014;46:44-45. |
| 145. | Wang PB, Zhao XF. [Efficacy and Safety Observation of Gegen Qinlian decoction Combined with Sulfasalazine Retention Enema in Treating Ulcerative Colitis]. Zhongguo Yiyao Zhinan. 2011;9:131-132. [DOI] [Full Text] |
| 146. | Wang JH. [Therapeutic efficacy observation of Ge Gen Qin Lian Tang in treating Ulcerative Colitis]. Xiandai Yiyao Weisheng. 2011;27:3462. |
| 147. | Wang QQ, Yu L, Zhen PC, Wang F. [Clinical Observation of 32 Cases of Chronic Nonspecific Ulcerative Colitis Treated with Ge Gen Qin Lian Tang Enema Combined with Oral Sulfasalazine Enteric-Coated Tablets]. Hebei Zhongyi. 2014;36:1023-1024. |
| 148. | Bian YY. [Analysis of the Efficacy of Combining Ge Gen Qin Lian Tang with Mesalazine in Treating Ulcerative Colitis]. Dangdai Yiyaolun Cong. 2015;13:182-183. |
| 149. | Nong YZ. [Clinical observation of 40 cases of Ulcerative Colitis treated with Gegen Qinlian decoction combined with probiotics]. Zhongguo Minzu Minjian Yiyao. 2017;26:106-107. |
| 150. | Wang CS. [Effects of Gegen Qinlian decoction Combined with Sulfasalazine on Inflammatory Responses in Patients with Ulcerative Colitis]. Guangming Zhongyi. 2018;33:1465-1467. |
| 151. | Xu AJ. [Clinical observation on the treatment of chronic non-specific Ulcerative Colitis with Ge Gen Qin Lian Tang enema combined with oral administration of enteric-coated sulfasalazine tablets]. Beifang Yaoxue. 2018;15:60-61. |
| 152. | Gong WL. [Effect of Gegen Qinlian Decoction Combined with Probiotics on Ulcerative Colitis and Sleep State]. Shijie Shuimian Yixue Zazhi. 2019;6:906-907. |
| 153. | Zhang N, Zhou XL, Liu S. [Clinical effects of Supplemented Gegen Qinlian Decoction combined with routine treatment on patients with severe ulcerative colitis due to Damp-heat Pattern]. Zhongchengyao. 2020;42:351-355. |
| 154. | Yang L, Wang XJ, Jin M, Li Y. [Efficacy and Partial Mechanism Study of Modified Gegen Qinlian decoction for Mild to Moderate Damp-Heat Accumulation Pattern Ulcerative Colitis]. Zhongguo Chufangyao. 2021;19:140-142. |
| 155. | Zhong QS, Lu XM. [Observation on the clinical effect of the modified Gegen Qinlian decoction plus mesalazine on ulcerative colitis]. Zhongyi Linchuang Yanjiu. 2021;13:123-125. |
| 156. | Gu LY, Luo M. [Clinical observation of modified Gegen Qinlian decoction and mesalazine on ulcerative colitis with damp-heat syndrome]. Shanxi Zhongyi. 2021;37:25-27. |
| 157. | Yu X. [Clinical effect Observation on Modified Gegen Qinlian Decoction Combined with Conventional Therapy in the Treatment of Severe Dampness-Heat Ulcerative Colitis]. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu. 2022;20:121-123. |
| 158. | Xu ZP, Wu YY, Cai EW. [Effect of Adjusted Gegen Qinlian Decoction on Ulcerative Colitis]. Zhongwai Yixue Yanjiu. 2022;20:30-33. [DOI] [Full Text] |
| 159. | Yang YL, Xiang LL, Chen PF, Zhu XL. [Effects of Modified Gegen QinlianDecoction on Immune Function and Intestinal Mucosal Barrier Function in Patients with Severe Damp-Heat Type of Ulcerative Colitis]. Liaoning Zhongyi Zazhi. 2023;50:92-95. |
| 160. | Xu YQ, Li ZJ, Zheng ML, Guan LY, Hu J, Tong CH, Zheng M. [Gegen Qinlian Decoction in the Treatment of Ulcerative Colitis]. Guangming Zhongyi. 2024;39:1911-1914. |
| 161. | Long XY, Gong YX, Tang YP. [Clinical Effect of Gegen Qinlian Decoction Combined with Mesalazine in Treatment of Damp-Heat Ulcerative Colitis of Large Intestine and Its Regulation of Intestinal Flora, Immune Function and Inflammation]. Zhonghua Zhongyiyaoxue Kan. 2024;42:118-121. [DOI] [Full Text] |
| 162. | Liu XY. [Clinical study on treatment of Ulcerative Colitis with Ge Gen Qin Lian Tang]. Neimenggu Zhongyiyao. 2024;43:54-56. [DOI] [Full Text] |
| 163. | Zhang XL, Liu JP, Bai HY, Du YR. [Clinical observation of Qinlian-lizhong decoction on recurrent chronic non-specific ulcerative colitis]. Hebei Zhongyi. 2017;39:1162-1165. |
| 164. | Zhang XL, Liu JP, Liu QQ, Du YR, Du MM. [Clinical observation on treatment of 60 cases of chronic recurrent ulcerative colitis with Qinlian Lizhong decoction]. Hebei Zhongyi. 2018;40:1463-1466, 1471. |
| 165. | Hu XD, Chen Y, Luo M, Li M, Yang ZL. [Clinical study on compound Qinbai granules retention enema for damp-heat descending pattern Ulcerative Colitis]. Yatai Chuantong Yiyao. 2016;12:148-149. |
| 166. | Yang MM, Luo WP, Li KY, Wang ZQ. [Clinical effect of retention enema with Qinbai granules in treatment of ulcerative colitis with damp-heat accumulation:an analysis of 37 cases]. Hunan Zhongyi Zazhi. 2017;33:10-12. [DOI] [Full Text] |
| 167. | Yang Q, Hu D. [Clinical Study on Huangqin Shaoyao Decoction Combined with Mesalazine Sustained-Release Tablets for Ulcerative Colitis of Spleen Deficiency and Dampness Accumula-tion Type]. Xin Zhongyi. 2025;57:39-43. [DOI] [Full Text] |
| 168. | Li M, Shi A, Pang H, Xue W, Li Y, Cao G, Yan B, Dong F, Li K, Xiao W, He G, Du G, Hu X. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J Ethnopharmacol. 2014;156:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 169. | Yi Y, Zhao Y, Li C, Zhang Y, Bin Y, Yuan Y, Pan C, Wang L, Liang A. Potential chronic liver toxicity in rats orally administered an ethanol extract of Huangqin (Radix Scutellariae Baicalensis). J Tradit Chin Med. 2018;38:242-256. [PubMed] |
| 170. | Zhang YY, Bai J, Xu MY, Zhang F, Bo CX. [Study on baicalin on liver and kidney toxicity in male rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021;39:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/