©The Author(s) 2025.
World J Gastroenterol. Dec 7, 2025; 31(45): 112720
Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.112720
Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.112720
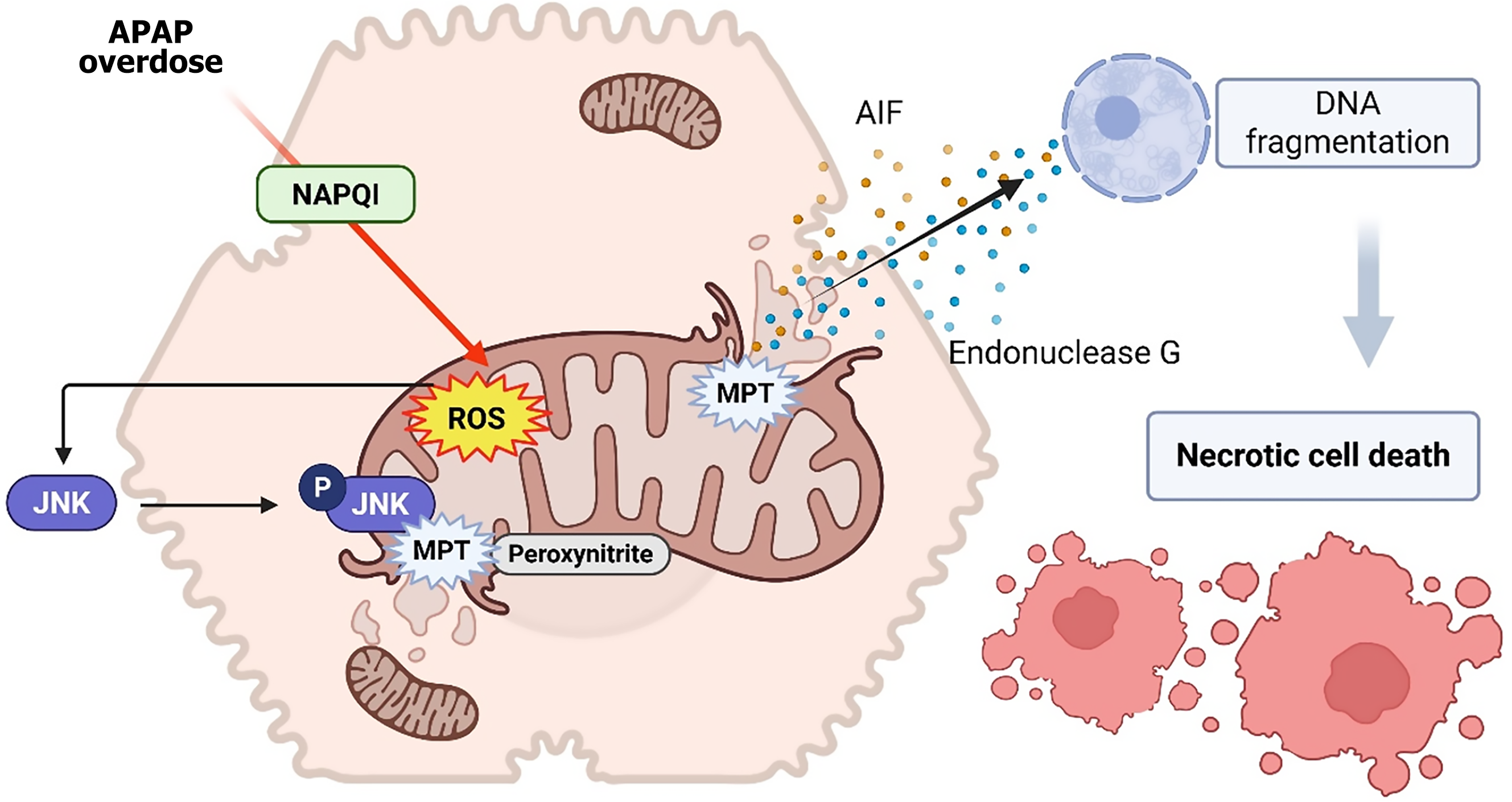
Figure 1 Hepatocellular mitochondrial injury and downstream death signaling triggered by acetaminophen overdose.
Following aceta
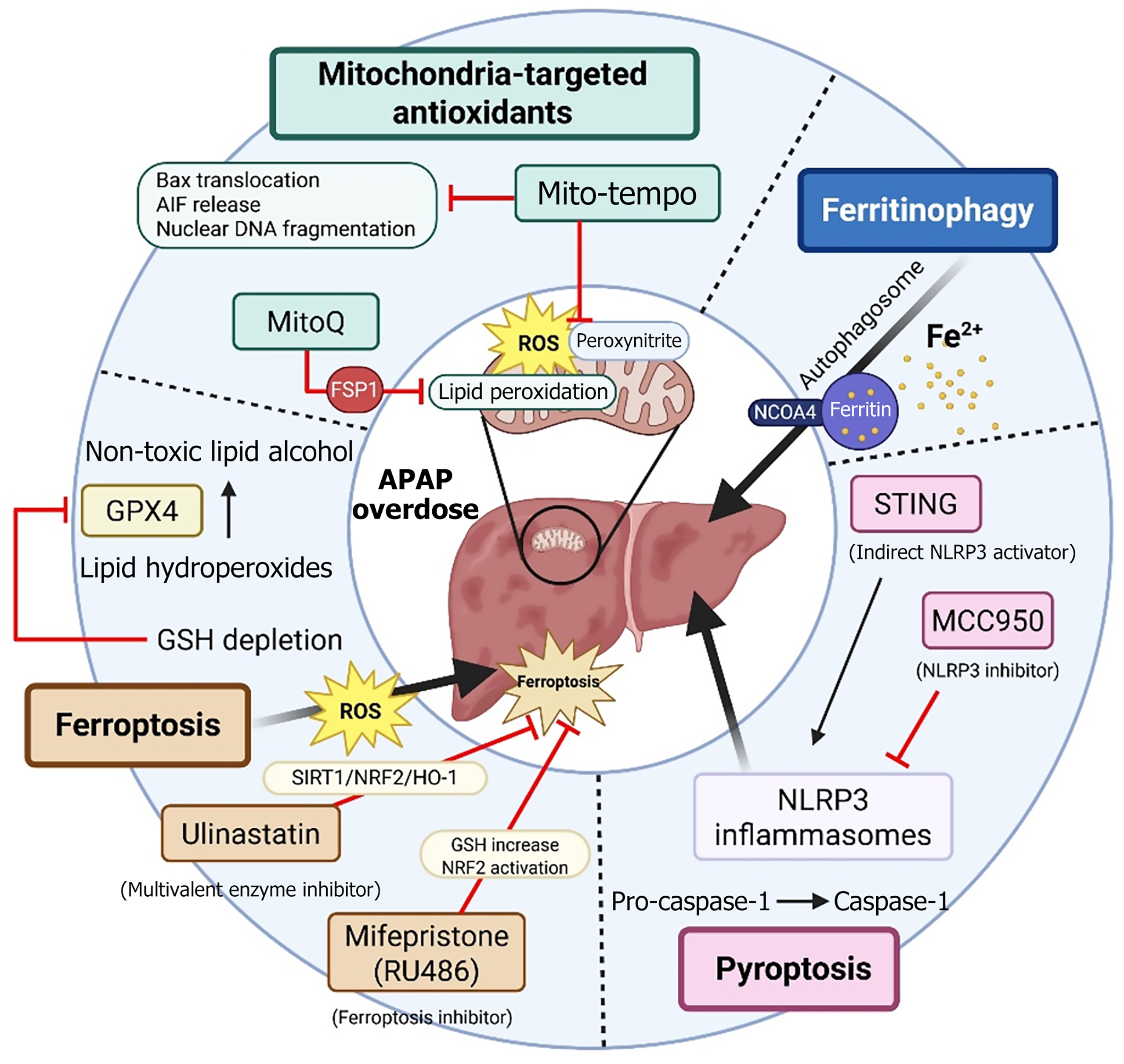
Figure 2 Targetable cell death and protective mechanisms in acetaminohphen-induced hepatotoxicity.
This figure provides an overview of the principal pathways involved in hepatocellular injury and protective responses in acetaminophen [N-acetyl-p-aminophenol (APAP)]-induced acute liver injury. Mitochondria-targeted antioxidants, such as Mito-Tempo and mitoquinone mesylate, safeguard hepatocytes by decreasing mitochondrial oxidative stress. Ferroptosis, which results from lipid peroxidation and is regulated by glutathione peroxidase 4, can be mitigated by inhibitors like Ulinastatin and mifepristone. Ferritinophagy, a process mediated by nuclear receptor coactivator 4 that leads to ferritin degradation, also promotes iron-dependent mitochondrial injury. Pyroptosis, initiated by activation of the NLR family pyrin domain containing 3 inflammasome and further enhanced via the stimulator of interferon genes pathway, can be reduced by using the selective inhibitor MCC950. Collectively, these mechanisms represent essential therapeutic opportunities aimed at maintaining hepatocyte viability in APAP-induced liver damage. AIF: Apoptosis-inducing factor; APAP: N-acetyl-p-aminophenol (Acetaminophen); FSP1: Ferroptosis suppressor protein 1; GPX4: Glutathione peroxidase 4; GSH: Glutathione; HO-1: Heme oxygenase-1; MitoQ: Mitoquinone mesylate; NCOA4: Nuclear receptor coactivator 4; NLRP3: NLR family pyrin domain containing 3; NRF2 Nuclear factor erythroid 2-related factor 2; ROS: Reactive oxygen species; SIRT1: Sirtuin 1; STING: Stimulator of interferon genes. This figure was created using BioRender.
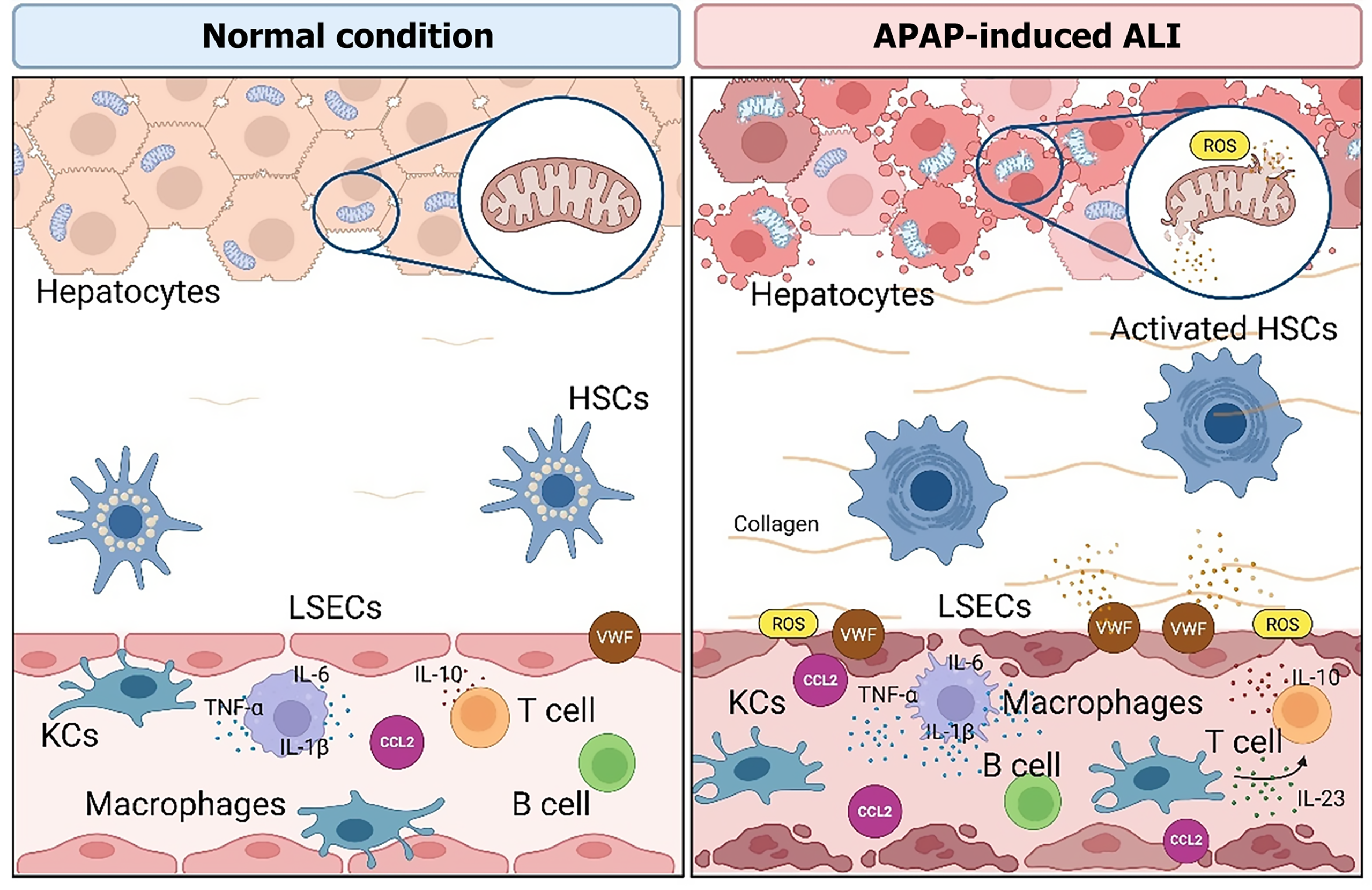
Figure 3 Overview of hepatic cell-type-specific responses in acetaminophen-induced acute liver injury.
This schematic depicts the coordinated actions of hepatic cell populations under both homeostatic conditions and following acetaminophen [N-acetyl-p-aminophenol (APAP)]-induced acute liver injury. An APAP overdose induces mitochondrial oxidative stress in hepatocytes, stimulates activation of hepatic stellate cells, elicits pro-inflammatory responses from Kupffer cells and monocyte-derived macrophages, and causes dysfunction of liver sinusoidal endothelial cells. The roles of adaptive immune cells, including T and B cells, are highlighted in the modulation of inflammation and tissue repair. This comprehensive illustration underlines the importance of multicellular dynamics governing both the progression and resolution phases of APAP-induced liver injury. APAP: N-acetyl-p-aminophenol (Acetaminophen); CCL2: Chemokine (C-C motif) ligand 2; HSCs: Hepatic stellate cells; IL: Interleukin; KCs: Kupffer cells; LSECs: Liver sinusoidal endothelial cells; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor-alpha; VWF: Von Willebrand factor. This figure was created using BioRender.
- Citation: Yang D, Kim B, Kim JW. Mechanistic insights into hepatic cell type-specific contributions to acetaminophen-induced acute liver injury. World J Gastroenterol 2025; 31(45): 112720
- URL: https://www.wjgnet.com/1007-9327/full/v31/i45/112720.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i45.112720