©The Author(s) 2025.
World J Gastroenterol. Nov 28, 2025; 31(44): 113793
Published online Nov 28, 2025. doi: 10.3748/wjg.v31.i44.113793
Published online Nov 28, 2025. doi: 10.3748/wjg.v31.i44.113793
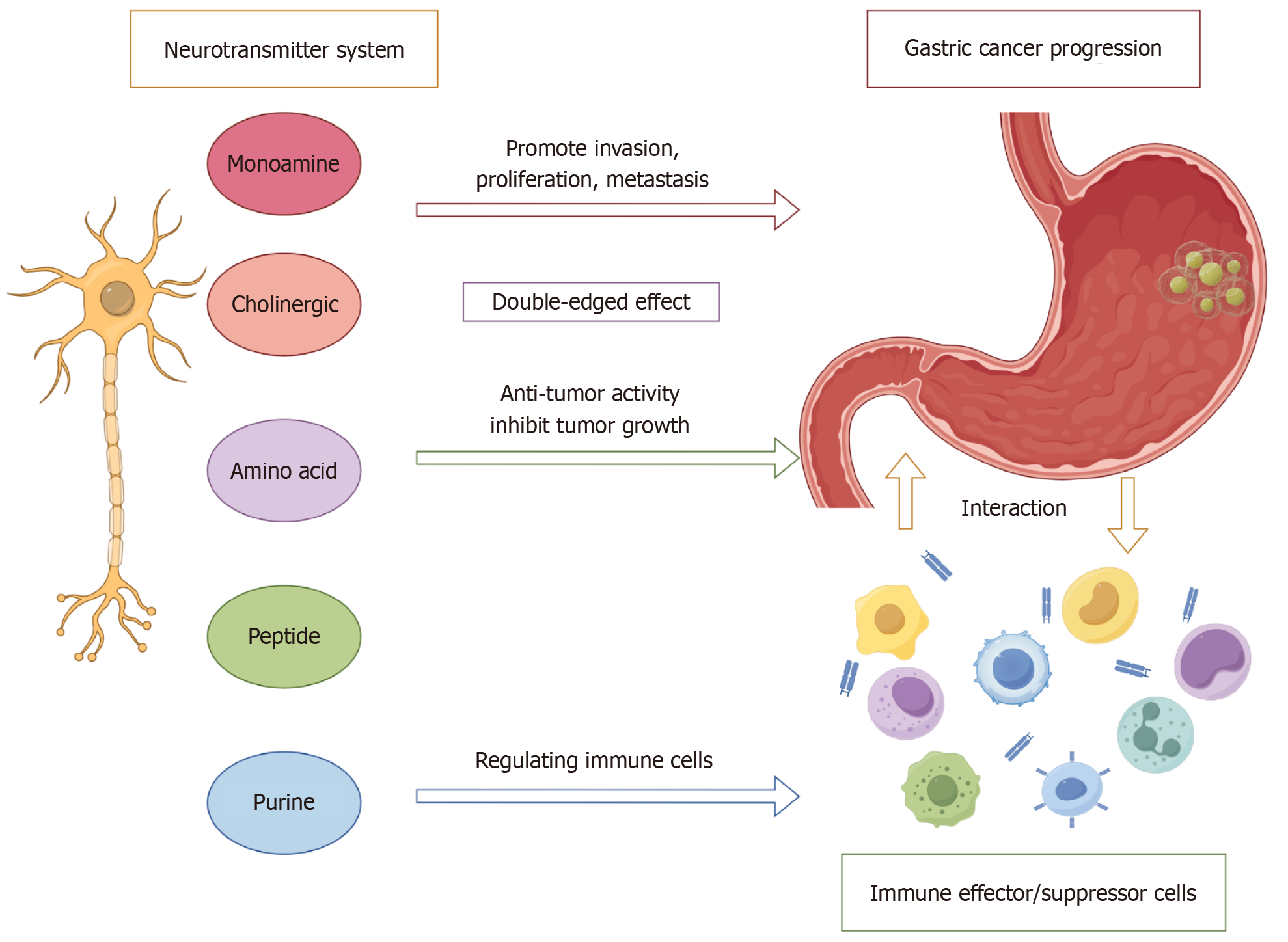
Figure 1 Introduction figure of the “neurotransmitters-immunity-tumors” axis.
Monoamines, cholinergic agents, amino acid derivatives, peptides, and purinergic neurotransmitters within the nervous system exert bidirectional effects on gastric carcinoma. Certain neurotransmitters promote cellular proliferation, invasive behavior, and metastatic progression, thereby facilitating tumor advancement. In contrast, others suppress oncogenic activity and restrain tumor growth. Moreover, neurotransmitters regulate the function of immune effector and suppressor cells, which dynamically interact with gastric cancer cells. The coordinated regulation of neurotransmitters, immune components, and malignant cells constitutes the so-called “neurotransmitter-immune-tumor” axis (by figdraw.com, Supplementary material).
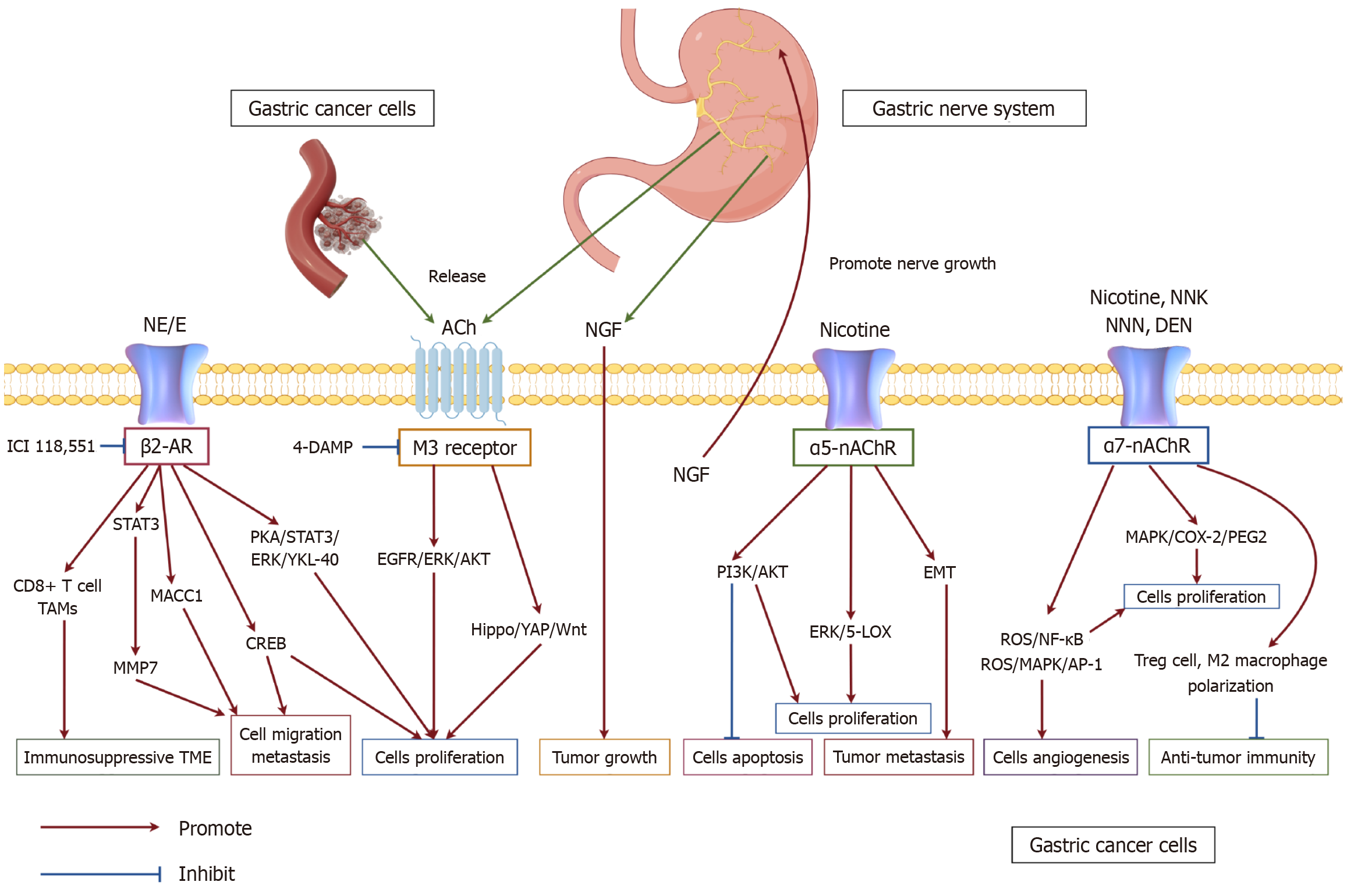
Figure 2 The interaction between gastric nerve system and gastric cancer.
Neurotransmitters released from the fibers of the gastric nervous system modulate gastric cancer progression by engaging specific receptors that control proliferation, invasion, migration, angiogenesis, and immune responses. Tumor cell growth and motility are enhanced by norepinephrine/epinephrine through theβ2-AR pathway, which signals via PKA/STAT3. Acetylcholine, produced both by gastric carcinoma cells and enteric ganglia, triggers oncogenic Hippo and EGFR signaling in malignant cells via M3 receptor activation. Nicotine and structurally related compounds facilitate gastric tumor development through α5-nAChR-driven PI3K or ERK pathways, while also promoting angiogenesis and suppressing antitumor immune activity via α7-nAChR-mediated ROS/MAPK signaling. Furthermore, gastric ganglia release nerve growth factor (NGF) to support tumor expansion, whereas gastric cancer cells in turn secrete NGF to stimulate neurite extension, forming a self-perpetuating neuro-tumoral feedback circuit (by figdraw.com, Supplementary material). Ach: Acetylcholine; NGF: Nerve growth factor; NE: Norepinephrine; E: Epinephrine.
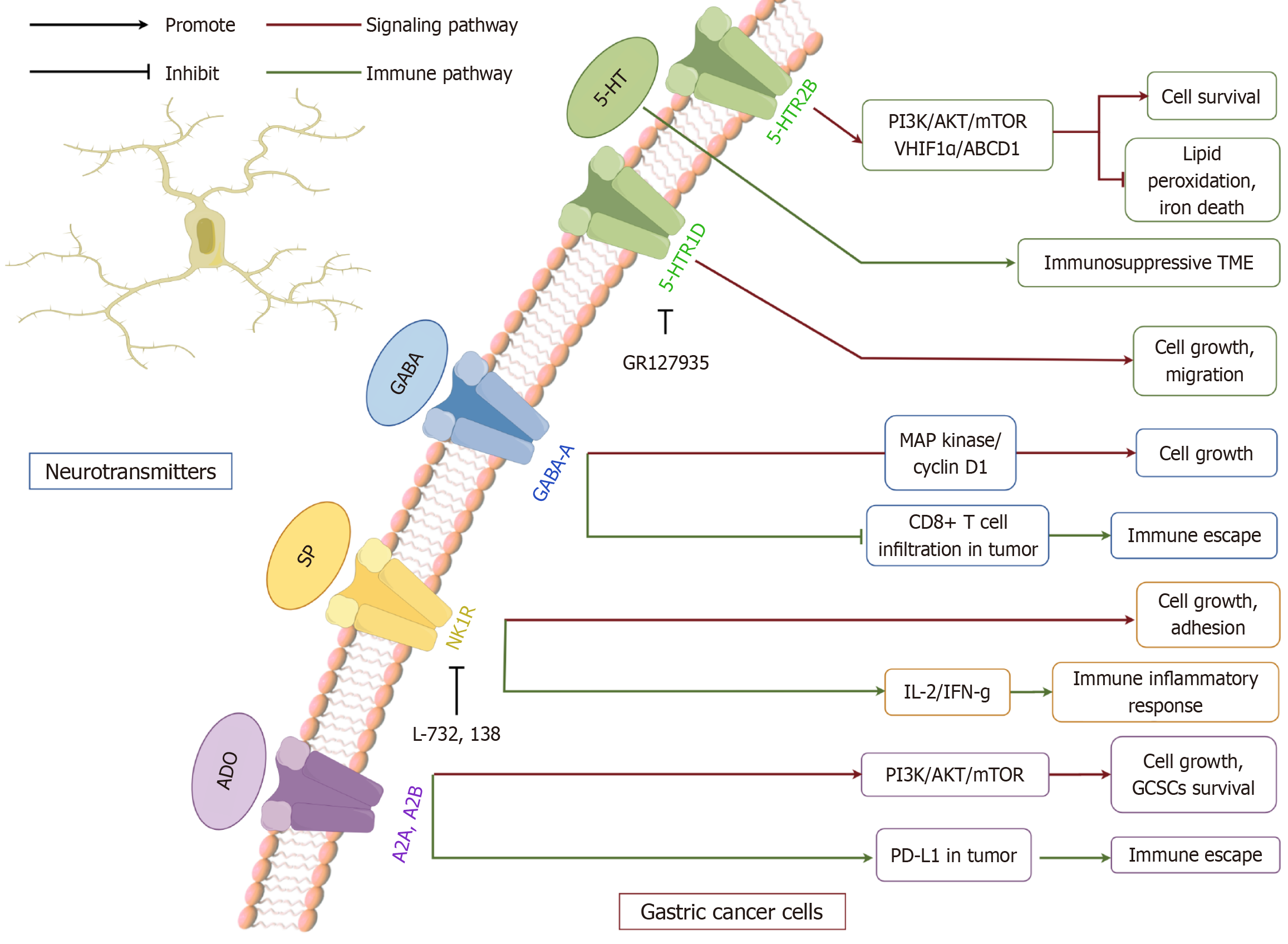
Figure 3 The interaction between serotonin, γ-aminobutyric acid, substance P, adenosine and gastric cancer.
Neurotransmitters from the monoamine, amino acid, peptide, and purine classes—namely serotonin (5-HT), γ-aminobutyric acid (GABA), substance P (SP), and adenosine (ADO)—interact with overexpressed receptors on gastric carcinoma cells, leading to the activation of distinct intracellular signaling networks. In particular, 5-HT engages HTR2B to stimulate the PI3K pathway, which supports cellular survival and proliferation while suppressing ferroptosis and apoptosis; through HTR1D, it further drives proliferative activity. GABA primarily connects with MAPK signaling, thereby fostering tumor expansion and metabolic reprogramming. SP triggers immune mediators such as IL-2, amplifying inflammatory responses. ADO, acting via A2A/B-dependent PI3K signaling, promotes tumor development and upregulates PD-L1 expression, enabling immune escape. Collectively, these ligand-receptor interactions initiate pathway-specific cascades that converge on hallmark tumor phenotypes, including growth, migration, invasion, and immune modulation, highlighting their diverse contributions to gastric carcinoma progression (by figdraw.com, Supplementary material). 5-HT: Serotonin; GABA: Γ-aminobutyric acid; SP: Substance P; ADO: Adenosine; TME: Tumor microenvironment.
- Citation: Liu YZ, Liu WX, Deng WH. Advances in the study of the relationship between neurotransmitters and gastric cancer. World J Gastroenterol 2025; 31(44): 113793
- URL: https://www.wjgnet.com/1007-9327/full/v31/i44/113793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i44.113793