©The Author(s) 2025.
World J Gastroenterol. Sep 14, 2025; 31(34): 110051
Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.110051
Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.110051
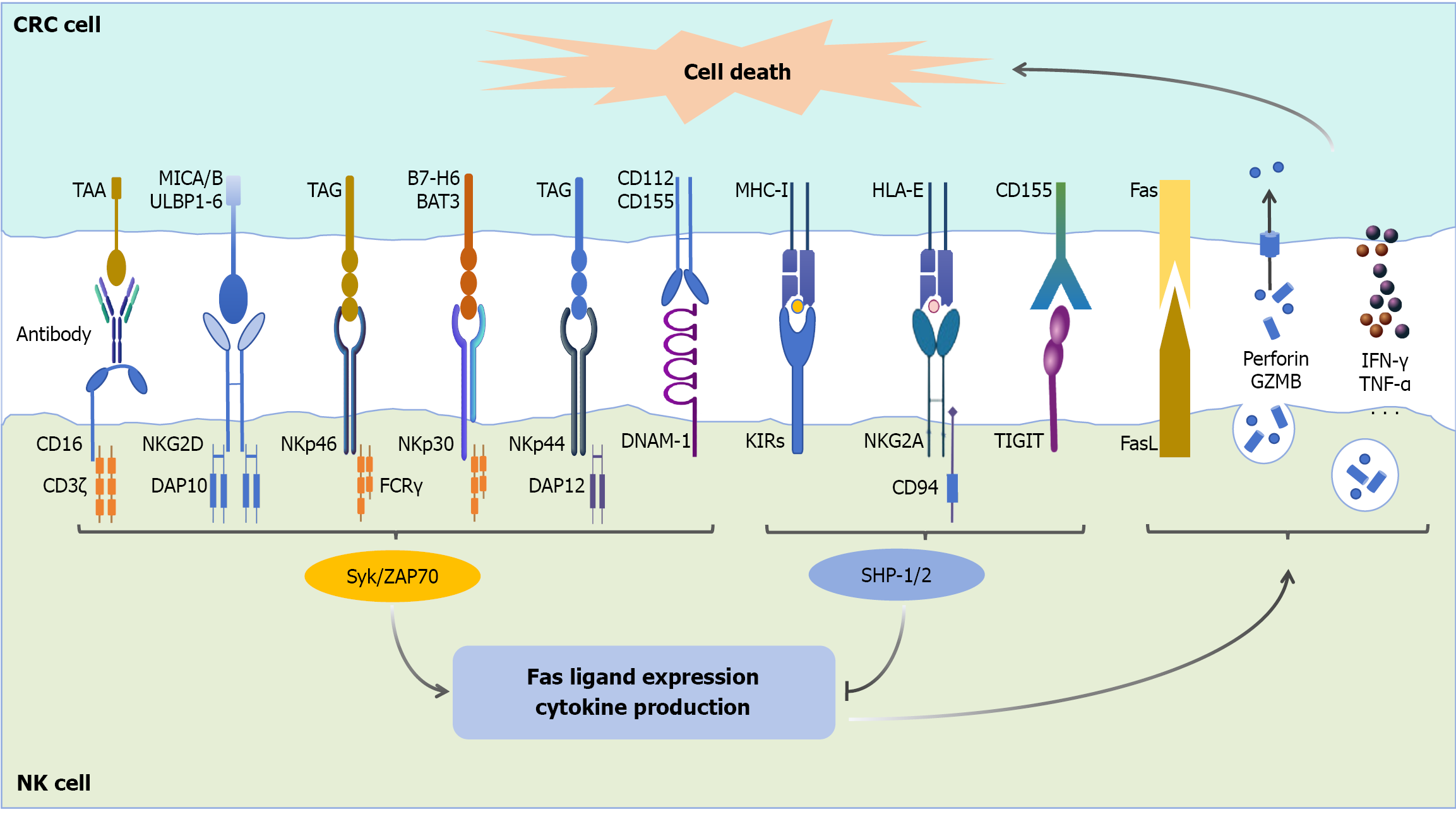
Figure 1 Receptor-mediated interactions between natural killer cells and colorectal cancer cells.
Natural killer (NK) cells engage with colorectal cancer (CRC) cells through a complex network of activating and inhibitory receptor-ligand interactions[16,17,22,25-36]. The activating receptors on NK cells, including CD16, NK group 2, member D, NKp46, NKp30, NKp44, and DNAX accessory molecule-1, recognize specific ligands expressed on CRC cells. These ligands include CD112, CD155, tumor-associated glycoproteins, human leukocyte antigen-B-associated transcript 3, BT-H6, major histocompatibility complex class I chain-related proteins A and B, UL16-binding proteins 1-6, and tumor-associated antigens. Upon receptor-ligand binding, NK cells become activated and initiate antitumor responses through multiple mechanisms: (1) The upregulation of Fas ligand; (2) The release of cytotoxic granules containing perforin and granzyme B; and (3) The secretion of pro-inflammatory cytokines such as interferon-γ and tumor necrosis factor-α. These effector functions collectively lead to CRC cell death. Conversely, inhibitory receptors on NK cells, including killer-cell immunoglobulin-like receptors, NK group 2, member A, and tyrosine-based inhibitory motif domain, interact with their corresponding ligands (major histocompatibility complex class I molecules, human leukocyte antigen-E, and CD155, respectively) on CRC cells. These interactions drive inhibitory signals that suppress NK cell activation and consequently impair their antitumor cytotoxicity. NK: Natural killer; CRC: Colorectal cancer; TAA: Tumor-associated antigen; MICA/B: Major histocompatibility complex class I chain-related proteins A and B; ULBP1-6: UL16-binding proteins 1-6; TAG: Tumor-associated glycoprotein; BAT3: Human leukocyte antigen-B-associated transcript 3; MHC-I: Major histocompatibility complex class I; HLA: Human leukocyte antigen; NKG2D: Natural killer group 2, member D; DAP10: DNAX-activating protein of 10 kDa; FCR: Fc receptor; DAP12: DNAX-activating protein of 12 kDa; DNAM-1: DNAX accessory molecule-1; KIRs: Killer-cell immunoglobulin-like receptors; TIGIT: Tyrosine-based inhibitory motif domain; FasL: Fas ligand; GZMB: Granules containing perforin and granzyme B; IFN-γ: Interferon-gamma; TNF-α: Tumor necrosis factor-α; Syk: Spleen tyrosine kinase; ZAP70: Zeta-chain associated protein 70; SHP: Src homology 2 domain-containing phosphatase.
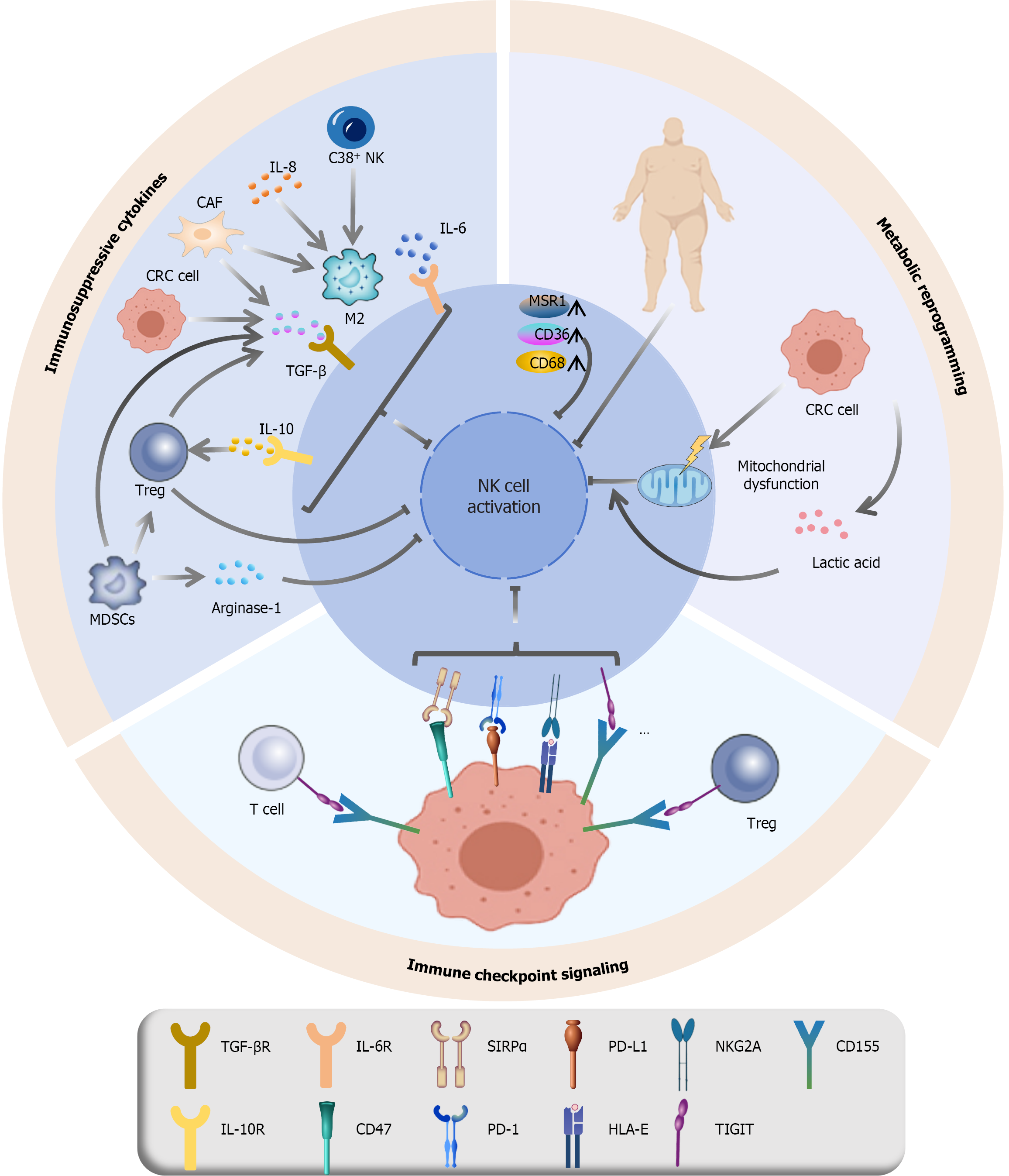
Figure 2 Suppressive mechanisms in the tumor microenvironment that impair natural killer cell function.
The antitumor activity of natural killer (NK) cells is compromised in the colorectal cancer microenvironment through multiple inhibitory mechanisms[49-53,55,57-65,70,77-80]: (1) Immunosuppressive cytokines: Cytokines such as interleukin (IL)-8, IL-6, transforming growth factor-β, and IL-10, which are secreted by cancer-associated fibroblasts, M2 macrophages, and colorectal cancer cells, directly or indirectly suppress NK cell cytotoxicity and effector functions; (2) Immune checkpoint signaling: Inhibitory immune checkpoint pathways, including like tyrosine-based inhibitory motif domain, programmed cell death protein 1, NK group 2, member A, and signal-regulatory protein alpha, along with their respective ligands, transmit suppressive signals that dampen NK cell activation and promote immune evasion; and (3) Metabolic dysregulation: Mitochondrial dysfunction, oxidative stress and apoptosis in NK cells are exacerbated by mitochondrial impairment. Another mechanism is lactate-mediated suppression, where high lactate levels in the tumor microenvironment induce mitochondrial stress, which further inhibits NK cell activity. Finally, lipid accumulation due to aberrant lipid metabolism is indicated by elevated macrophage scavenger receptor 1, CD36, and CD68, which impairs NK cell function and reduces tumor-killing capacity. NK: Natural killer; IL: Interleukin; CAF: Cancer-associated fibroblast; CRC: Colorectal cancer; MSR1: Macrophage scavenger receptor 1; TGF: Transforming growth factor; Treg: Regulatory T cell; MDSC: Myeloid-derived suppressor cell; SIRPα: Signal-regulatory protein alpha; PD-L1: Programmed death ligand 1; NKG2A: Natural killer group 2, member A; PD-1: Programmed cell death protein 1; HLA: Human leukocyte antigen; TIGIT: Tyrosine-based inhibitory motif domain.
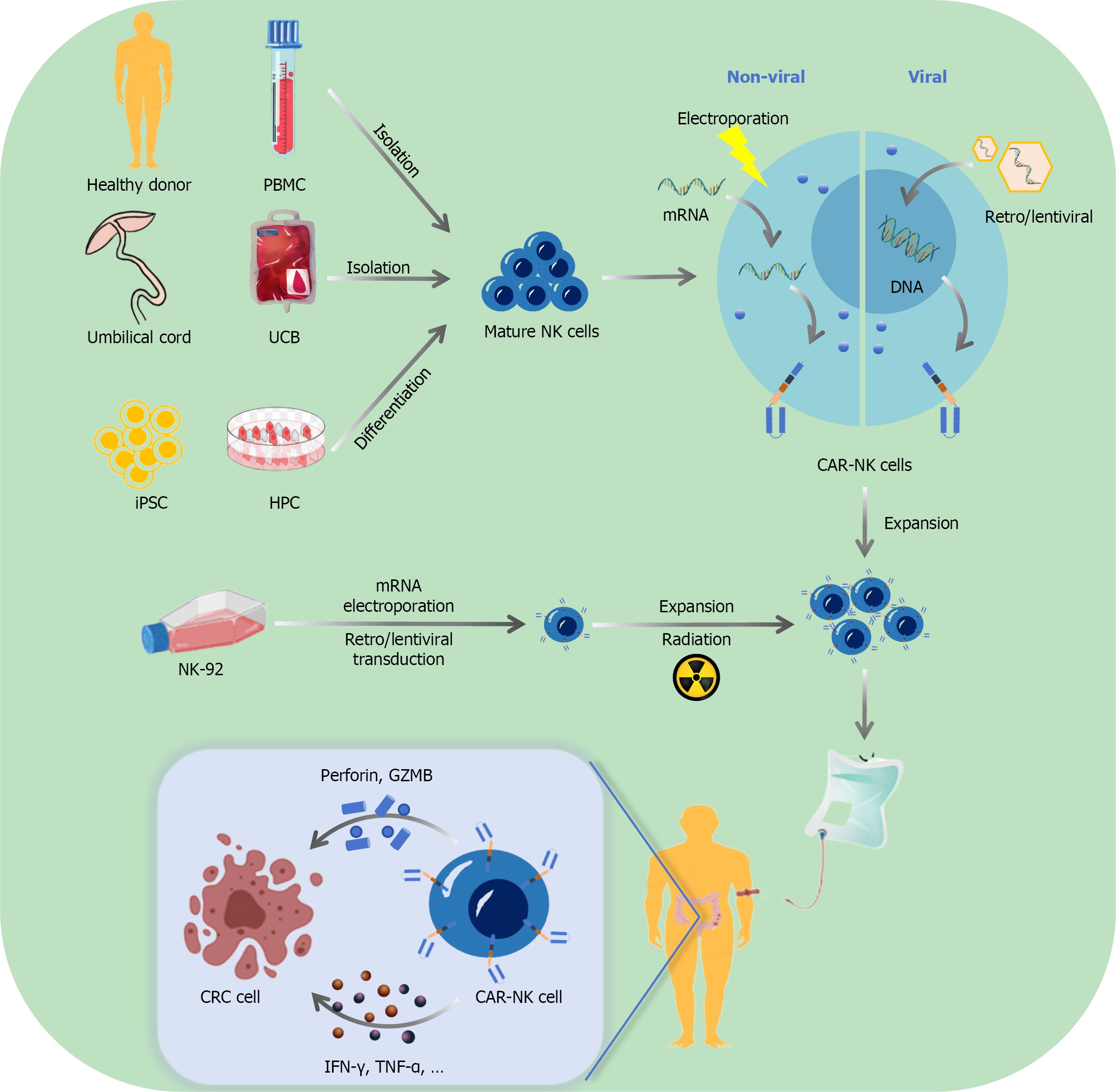
Figure 3 Generation and mechanism of chimeric antigen receptor-natural killer cells in colorectal cancer therapy[16,131-135,152,153,159,160].
Cell sources for chimeric antigen receptor (CAR)-natural killer (NK) generation: CAR-NK cells can be derived from multiple sources, including peripheral blood mononuclear cells from healthy donors, umbilical cord blood, hematopoietic progenitor cells differentiated from induced pluripotent stem cells, and the immortalized NK-92 cell line. Genetic modification methods: Two primary approaches are used for CAR delivery, including nonviral methods (electroporation for transient mRNA transfection), and viral methods (retroviral or lentiviral vectors for stable DNA integration). Cell processing and expansion: Following genetic modification, the cells undergo ex vivo expansion to achieve therapeutic quantities as well as radiation treatment (for certain cell types derived from NK-92) to ensure safety. Therapeutic mechanism in colorectal cancer: When infused into patients, CAR-NK cells recognize and bind to tumor-associated antigens on colorectal cancer cells, and initiate cytotoxic responses through the release of perforin and granules containing perforin and granzyme B and the secretion of pro-inflammatory cytokines, thereby effectively eliminating tumor cells while minimizing off-target effects. PBMC: Peripheral blood mononuclear cell; UCB: Umbilical cord blood; NK: Natural killer; CAR: Chimeric antigen receptor; iPSC: Induced pluripotent stem cell; HPC: Hematopoietic progenitor cell; GZMB: Granules containing perforin and granzyme B; CRC: Colorectal cancer; IFN-γ: Interferon-gamma; TNF-α: Tumor necrosis factor-α.
- Citation: Zhang XJ, Yu Y, Li JY, Yan YZ, Jiang SS, Zhang Y, Fei Q, Zhang YR, Zhao YX, Guo L, Lv J, Zhao HP. Natural killer cell-based immunotherapies for colorectal cancer: Current strategies, challenges, and future perspectives. World J Gastroenterol 2025; 31(34): 110051
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/110051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.110051