©The Author(s) 2025.
World J Gastroenterol. Sep 7, 2025; 31(33): 108653
Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.108653
Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.108653
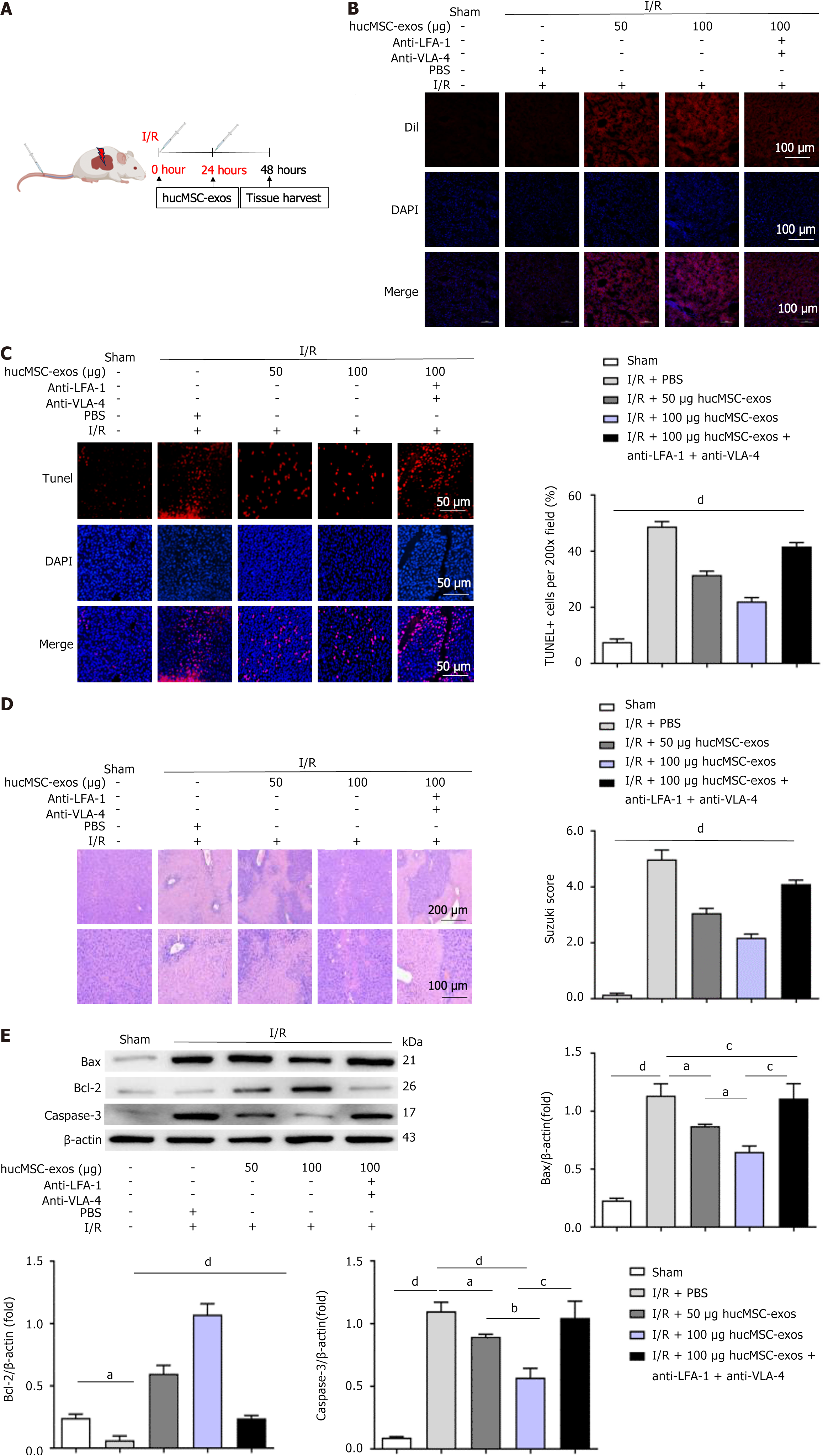
Figure 1 Human umbilical cord mesenchymal stem cell-derived exosomes alleviate hepatic ischaemia-reperfusion injury in mice.
A: Schematic of the experimental design for the mouse model of hepatic ischaemia-reperfusion injury (HIRI). The mice were subjected to occlusion of hepatic blood flow in the left lateral lobe and left medial lobe for 1 hour followed by reperfusion. At the start of reperfusion, the mice were intravenously injected with 50 μg of human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-exos), 100 μg of hucMSC-exos, or 100 μg of hucMSC-exos treated with anti-very late antigen-4 and anti-lymphocyte function-associated antigen-1 antibodies. Phosphate-buffered saline (100 μL) was used as a control for HIRI. Liver tissue samples were collected 48 hours after HIRI induction; B: Cellular uptake of DiI-labelled hucMSC-exos in liver tissue. Scale bars: 100 μm; C: TUNEL staining. Scale bars: 50 μm; D: Hematoxylin and eosin staining. Scale bars are 200 μm (top panel) and 100 μm (bottom panel); E: Western blot analysis of apoptosis-related proteins in liver tissues. I/R: Ischaemia/reperfusion; hucMSC-exos: Human umbilical cord mesenchymal stem cell-derived exosomes; LFA-1: Lymphocyte function-associated antigen-1; VLA-4: Very late antigen-4; PBS: Phosphate-buffered saline; DiI: 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DAPI: 4’,6-diamidino-2-phenylindole; TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling; Bax: B-cell lymphoma-2-associated X protein; Bcl-2: B-cell lymphoma-2; Caspase-3: Cysteinyl aspartate specific proteinase-3. aP < 0.05, bP < 0.01, cP < 0.001, and dP < 0.0001.
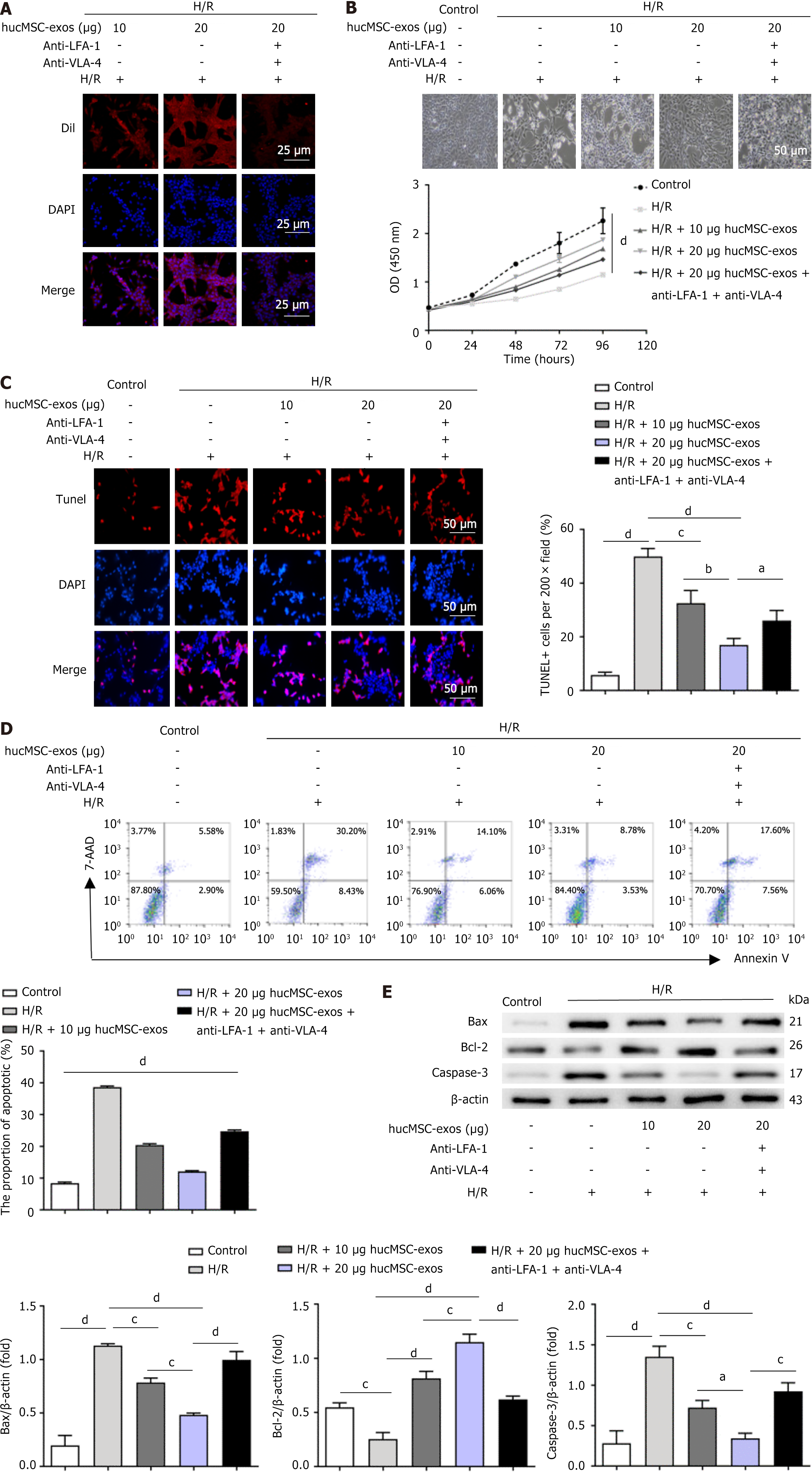
Figure 2 Human umbilical cord mesenchymal stem cell-derived exosomes attenuate apoptosis in LO2 cells following hypoxia/re
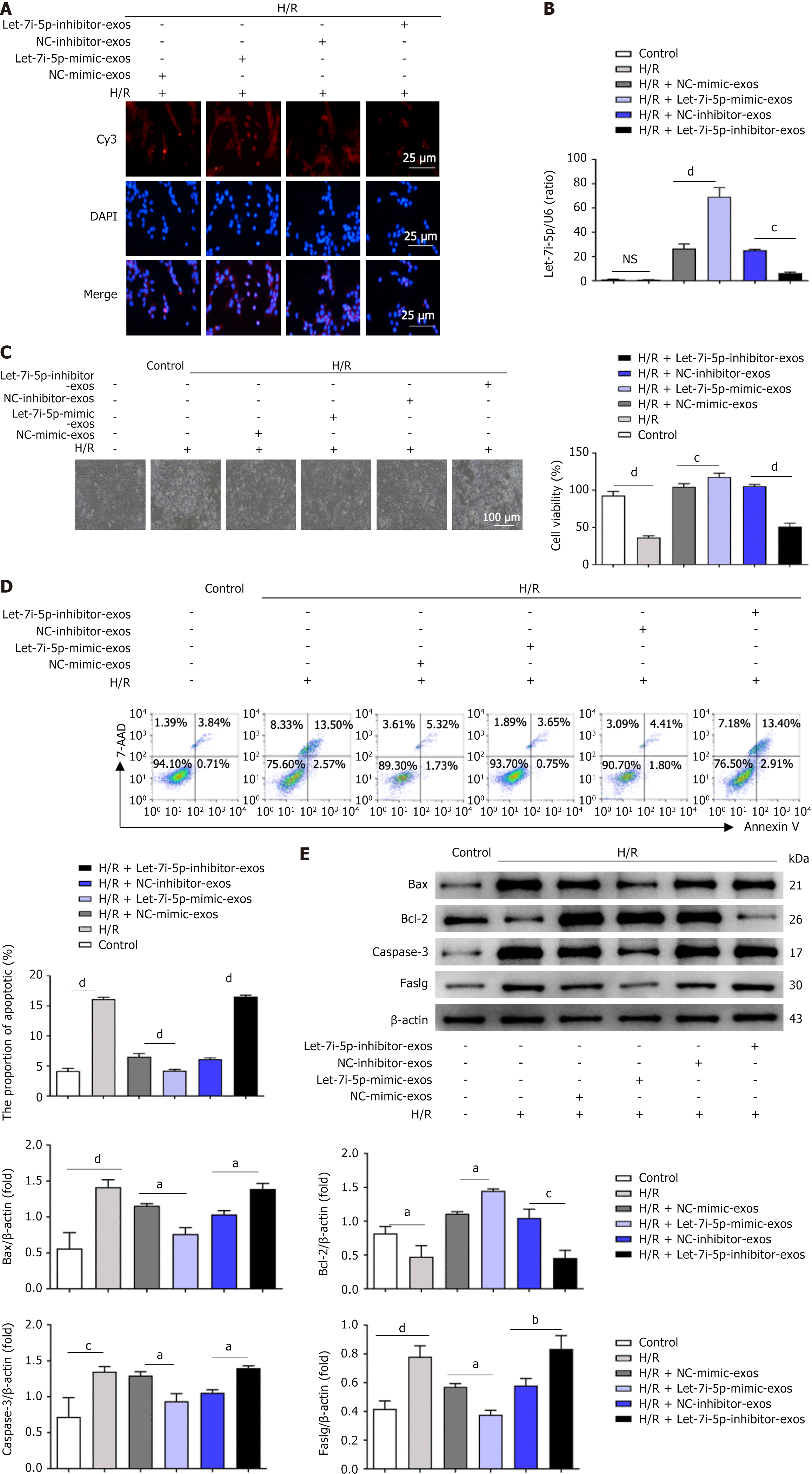
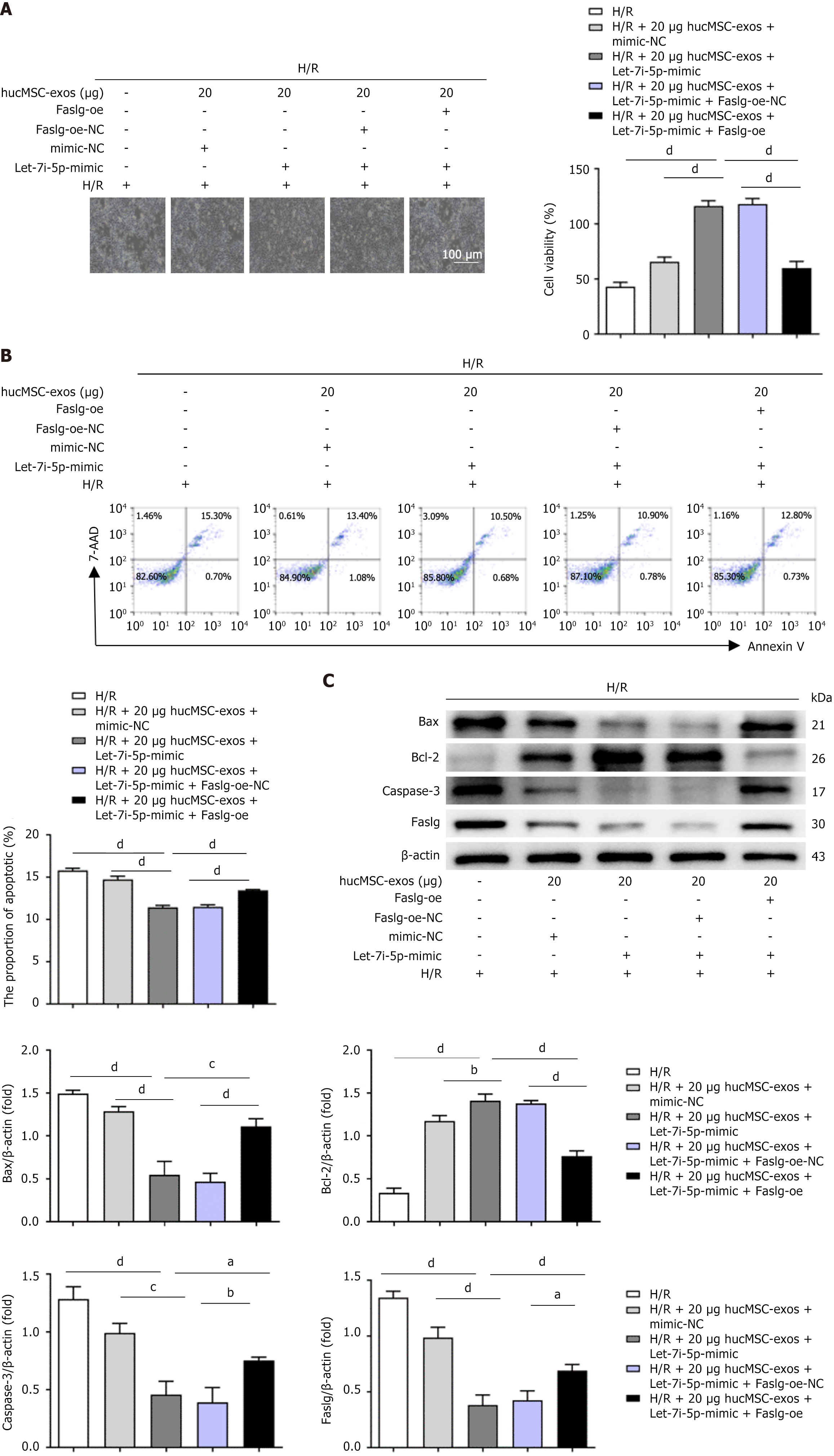
Figure 3 Role of let-7i-5p in the effects of human umbilical cord mesenchymal stem cell-derived exosomes on the apoptosis of LO2 cells after hypoxia/reoxygenation injury.
A: Representative fluorescence images showing the uptake of transfected human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-exos) by LO2 cells after hypoxia/reoxygenation (H/R) injury. Scale bars: 25 μm; B: After the coincubation of H/R-treated LO2 cells with transfected hucMSC-exos, quantitative real-time polymerase chain reaction was used to detect the expression levels of let-7i-5p in LO2 cells; C: Representative light microscopy images of H/R-treated LO2 cells coincubated with hucMSC-exos overexpressing or inhibiting let-7i-5p. Scale bars: 100 μm; D: Flow cytometry was used to detect the apoptosis rate of LO2 cells after the coincubation of transfected hucMSC-exos with H/R-treated LO2 cells; E: Western blot analysis of changes in the expression levels of apoptosis-related protein markers in LO2 cells after the coincubation of transfected hucMSC-exos with H/R-treated LO2 cells. H/R: Hypoxia/reoxygenation; DAPI: 4’,6-diamidino-2-phenylindole; Cy3: Cyanine3; NC: Negative control; Bax: B-cell lymphoma-2-associated X protein; Bcl-2: B-cell lymphoma-2; Caspase-3: Cysteinyl aspartate specific proteinase-3; Faslg: Factor-related apoptosis ligand; 7-AAD: 7-aminoactinomycin D. aP < 0.05, bP < 0.01, cP < 0.001, and dP < 0.0001.
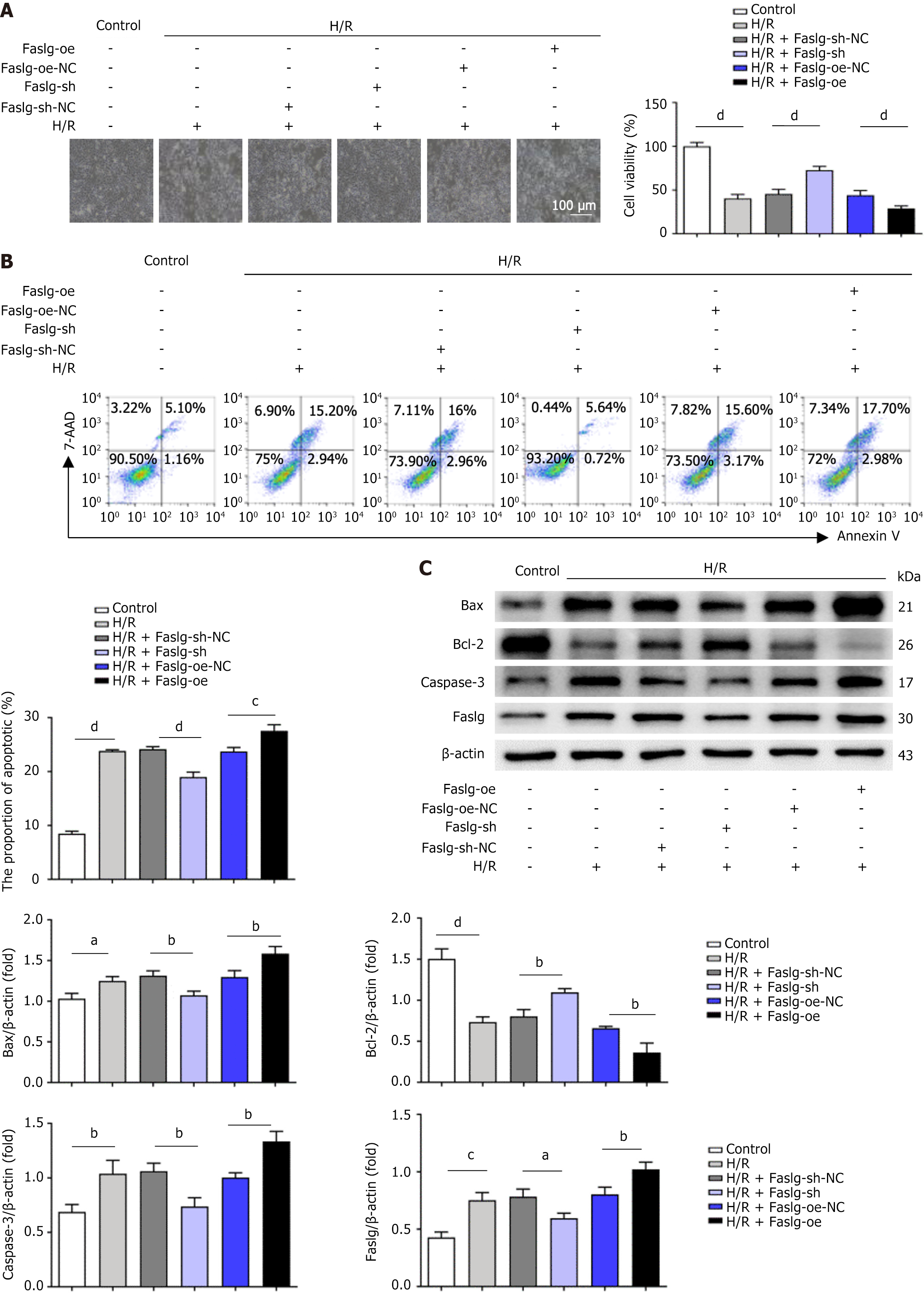
Figure 4 Role of factor-related apoptosis ligand-mediated let-7i-5p in human umbilical cord mesenchymal stem cell-derived exosomes in regulating the apoptosis of LO2 cells after hypoxia/reoxygenation injury.
A: Representative light microscopy images of LO2 cells infected with lentiviral vectors after hypoxia/reoxygenation (H/R) treatment. Scale bars: 100 μm; B: Flow cytometry was used to detect the apoptosis rate of LO2 cells after lentiviral vector infection followed by H/R treatment; C: Western blot analysis was performed to detect the protein levels of apoptosis-related markers in LO2 cells after factor-related apoptosis ligand overexpression or factor-related apoptosis ligand knockdown followed by H/R treatment. H/R: Hypoxia/reoxygenation; Faslg-oe: Lentiviral vector overexpressing factor-related apoptosis ligand; Faslg-sh: Factor-related apoptosis ligand short hairpin RNA, lentiviral RNA interference vector-mediated factor-related apoptosis ligand; NC: Negative control; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-associated X protein; Caspase-3: Cysteinyl aspartate specific proteinase-3; Faslg: Factor-related apoptosis ligand; 7-AAD: 7-aminoactinomycin D. aP < 0.05, bP < 0.01, cP < 0.001, and dP < 0.0001.
Figure 5 Let-7i-5p in human umbilical cord mesenchymal stem cell-derived exosomes affects the level of LO2 cell apoptosis after hypoxia/reoxygenation injury by regulating the expression of factor-related apoptosis ligand.
A: Representative light microscopy images of LO2 cells after hypoxia/reoxygenation (H/R) treatment in the cell rescue experiment. Scale bars: 100 μm; B: Flow cytometry analysis of the apoptosis rate of LO2 cells after H/R treatment in the cell rescue experiment; C: Western blot analysis of the protein levels of apoptosis-related markers in LO2 cells after H/R treatment in the cell rescue experiment. H/R: Hypoxia/reoxygenation; hucMSC-exos: Human umbilical cord mesenchymal stem cell-derived exosomes; Faslg-oe: Lentiviral vectors overexpressing factor-related apoptosis ligand; NC: Negative control; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-associated X protein; Caspase-3: Cysteinyl aspartate specific proteinase-3; Faslg: Factor-related apoptosis ligand. aP < 0.05, bP < 0.01, cP < 0.001, and dP < 0.0001.
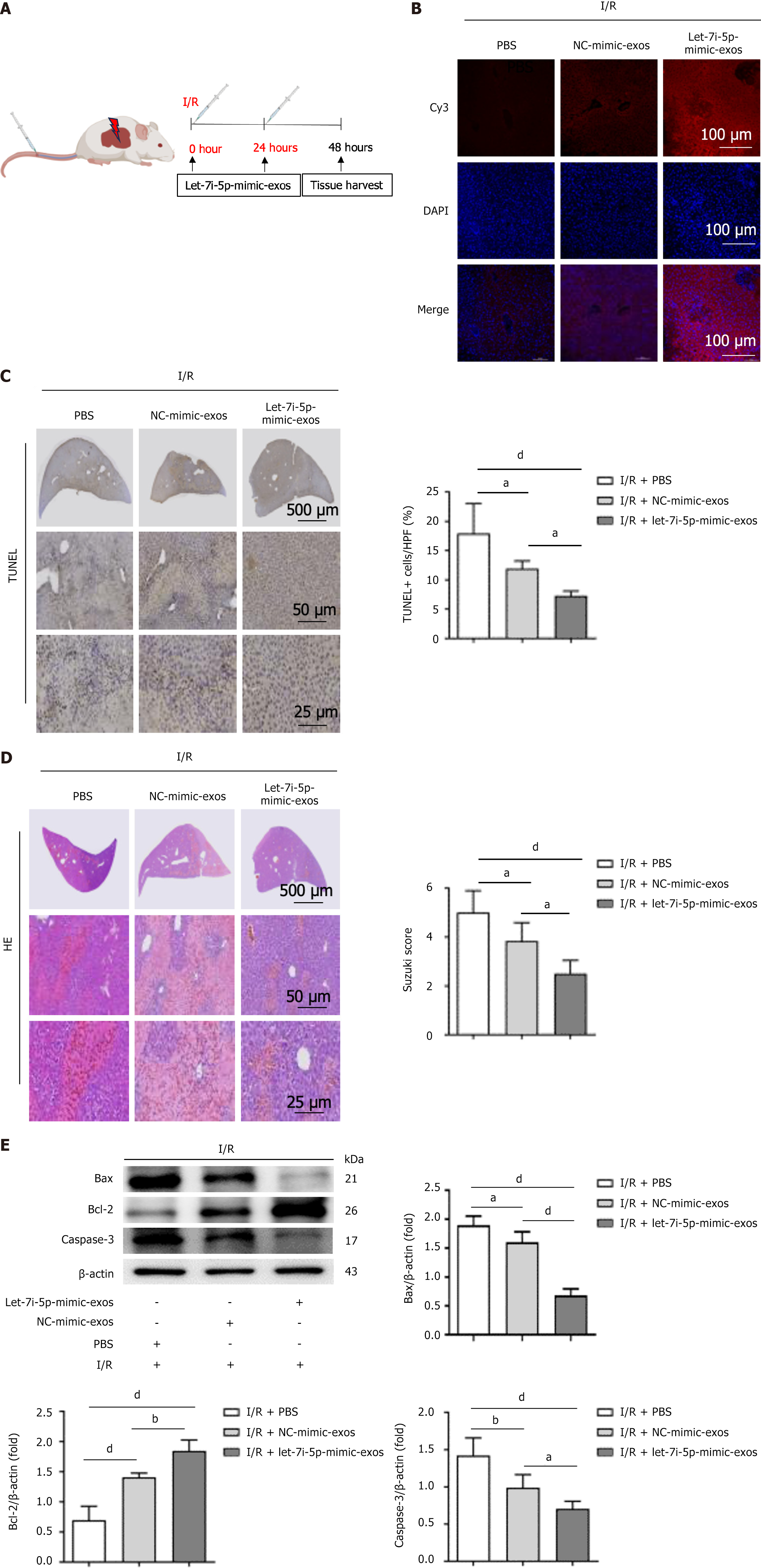
Figure 6 The let-7i-5p mimic enhances the protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on hepatic ischaemia-reperfusion injury in mice.
A: A schematic of the experimental design of the hepatic ischaemia-reperfusion injury (HIRI) mouse model is shown. The mice were subjected to 1 hour of occlusion of hepatic blood flow in the left lateral lobe and left medial lobe followed by reperfusion. At the beginning of reperfusion, 100 μg of cyanine3 (Cy3)-let-7i-5p-mimic-exosomes (exos) or Cy3-negative control-mimic-exos was injected via the tail vein. For the HIRI control group, 100 μL of sterile phosphate-buffered saline was used. Liver tissue samples were collected 48 hours after HIRI induction; B: The uptake of Cy3-labelled human umbilical cord mesenchymal stem cell-derived exos in liver tissue is shown. Scale bars: 100 μm; C: TUNEL staining of liver tissues. Scale bars: 500 μm (top panel), 50 μm (middle panel), and 25 μm (bottom panel); D: Haematoxylin and eosin staining. Scale bars: 500 μm (top panel), 50 μm (middle panel), and 25 μm (bottom panel); E: Western blot analysis was used to detect the protein levels of apoptosis-related markers in liver tissue. I/R: Ischaemia/reperfusion; exos: Exosomes; Cy3: Cyanine3; DAPI: 4’,6-diamidino-2-phenylindole; PBS: Phosphate-buffered saline; NC: Negative control; TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling; HE: Haematoxylin and eosin; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-associated X protein; Caspase-3: Cysteinyl aspartate specific proteinase-3. aP < 0.05, bP < 0.01, and dP < 0.000.
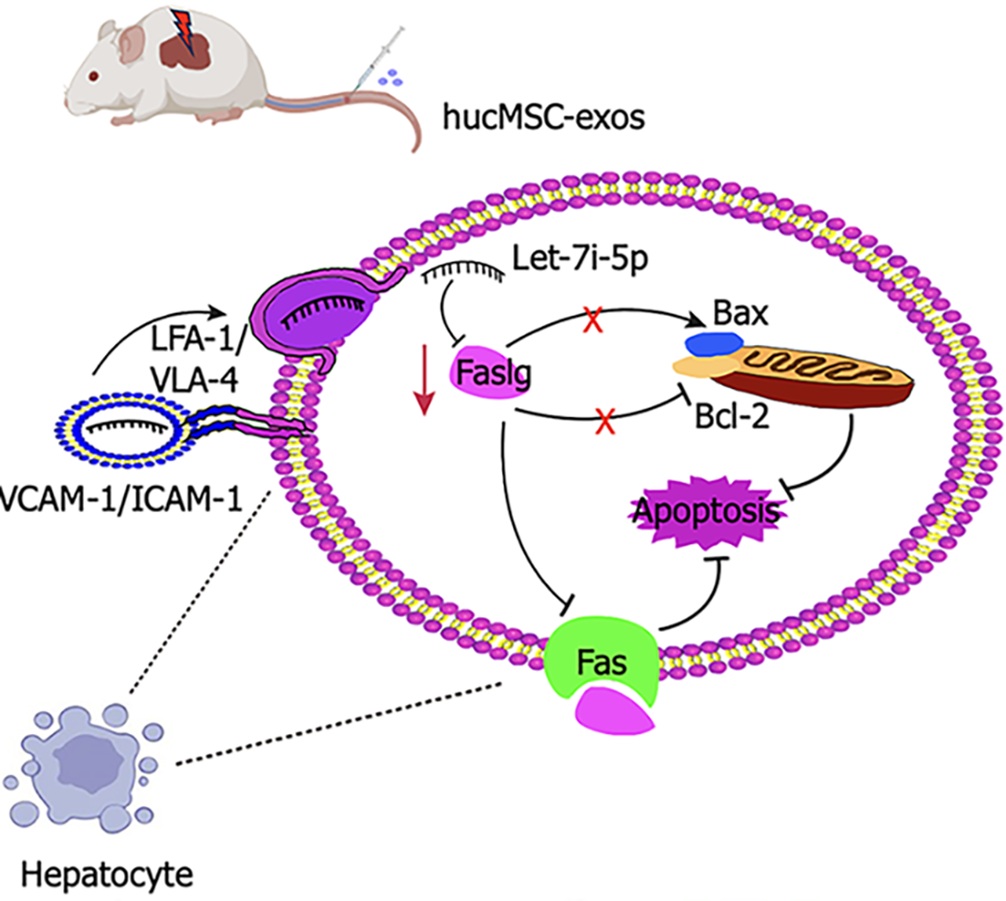
Figure 7 Schematic illustration of the therapeutic mechanism of human umbilical cord mesenchymal stem cell-derived exosomes in hepatic ischaemia-reperfusion injury.
Hepatic ischaemia-reperfusion injury induces hepatocyte damage and triggers apoptosis. Human umbilical cord mesenchymal stem cell-derived exosomes target the injured liver via very late antigen-4/Lymphocyte function-associated antigen-1-mediated adhesion interactions. The therapeutic effects of these exosomes primarily involve the inhibition of the death receptor-mediated extrinsic apoptotic pathway through the downregulation of factor-related apoptosis ligand expression; and the inhibition of the mitochondrial-mediated intrinsic apoptotic pathway by modulating the B-cell lymphoma-2/B-cell lymphoma-2-associated X protein expression ratio. This process synergistically suppresses hepatocyte apoptosis. HucMSC-exos: Human umbilical cord mesenchymal stem cell-derived exosomes; VLA-4: Very late antigen-4; LFA-1: Lymphocyte function-associated antigen-1; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-associated X protein; Fas: Factor-related apoptosis; Faslg: Factor-related apoptosis ligand; VCAM-1: Vascular cell adhesion molecule-1; ICAM-1: Intercellular cell adhesion molecule-1.
- Citation: Gao Y, He M, Bian CW, Yu R, Luo JJ, Xiang YM, Yang YX, Huang HF, Zeng Z. Exosomes derived from human umbilical cord mesenchymal stem cells attenuate hepatic ischaemia-reperfusion injury via the let-7i-5p/Faslg axis. World J Gastroenterol 2025; 31(33): 108653
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/108653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.108653