Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.108872
Revised: June 27, 2025
Accepted: August 12, 2025
Published online: September 7, 2025
Processing time: 124 Days and 18.5 Hours
Second-line treatment of Crohn’s disease (CD) commonly involves immunosuppressants such as azathioprine, mercaptopurine, or methotrexate (MTX), used either alone or in combination.
To investigate the current use of MTX among French gastroenterologists.
An online questionnaire was distributed between March and August 2023 to 116 French gastroenterologists managing CD. A total of 87 respondents completed the survey and were included in the analysis.
Respondents reported a mean annual caseload of 140 CD patients (median: 50). Overall, 71% prescribed MTX, predominantly in injectable form (92%), either as monotherapy or in combination with biologics or cyclosporin. MTX was prescribed for mild-to-moderate CD by 64% of respondents, and for severe CD by 58%, often in combination with an anti-tumor necrosis factor agent (89% and 94%, respectively). Injectable MTX was favored (84%) in specific clinical scenarios: Patients with articular manifestations (77%), Epstein-Barr virus-negative status (65%), or aged over 65 years (58%). Among the 29% of non-prescribers, the primary reason cited was lack of familiarity with MTX use (60%). Both prescribers and non-prescribers expressed the need for clearer guidelines and real-world data to support MTX use.
Regardless of prescribing habits, most respondents had a favorable opinion of MTX and recognized its good long-term safety profile. French learned societies and medical associations should provide consensus guidelines on MTX use, supported by validated real-world safety and effectiveness data.
Core Tip: This practice survey among French gastroenterologists reveals a growing confidence in methotrexate (MTX) for managing Crohn’s disease (CD), particularly in combination with biologics for specific patient profiles such as those with joint involvement or Epstein-Barr virus negativity. While MTX is often underutilized compared to azathioprine, respondents acknowledged its favorable long-term safety profile. The study highlights variability in prescribing habits and underlines the need for clearer clinical guidelines and more real-world evidence to support optimal use of MTX in CD.
- Citation: Bonnaud G, Becker J, Chebbah M, Courbeyrette A, Faure P. Methotrexate in the management of Crohn’s disease: A practice survey of gastroenterologists in France. World J Gastroenterol 2025; 31(33): 108872
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/108872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.108872
Crohn’s disease (CD) is a chronic inflammatory bowel disorder (IBD) of unknown etiology, with a rising global prevalence worldwide now exceeding 0.3%. It imposes a significant burden on patients[1,2]. The primary goal of CD management is to achieve and maintain remission through a comprehensive, multidimensional approach, as outlined in recent guidelines, including the selecting therapeutic targets in inflammatory bowel disease initiative by the International Organization for the Study of IBD in 2021[3], the European Crohn’s and Colitis Organization (ECCO) in 2024[4], and the National Institute for Health and Care Excellence (NICE) in 2024[5]. Remission in CD is defined by several complementary criteria: Clinical remission is based on symptom relief, assessed using tools such as the patient reported outcomes 2 score (minimal abdominal pain and stool frequency) or a Harvey-Bradshaw index < 5; Biochemical remission requires normalization of inflammatory markers, including C-reactive protein (CRP) and fecal calprotectin (< 250 μg/g); Endoscopic remission corresponds to mucosal healing, with a simple endoscopic score for CD (SES-CD) ≤ 2 or CD endoscopic index of severity (CDEIS) < 3 and no visible ulcers. In addition, improving health-related quality of life and minimizing disability remain central therapeutic targets. Transmural healing, assessed through cross-sectional imaging, is increasingly recognized as an adjunctive marker of deep remission, improved long-term outcomes and improve overall patient well-being[3]. Over the past two decades, therapeutic options for CD have expanded significantly, particularly with the introduction of biologic therapies[6]. For mild-to-moderate disease, first-line treatment generally involves anti-inflammatory agents such as corticosteroids (e.g., budesonide)[3-5,7]. Immunosuppressants, including azathioprine (AZA), mercaptopurine, thioguanine, and methotrexate (MTX), play a key role in managing more complex cases and preventing disease progression. Typically used as second-line therapy, immunosuppressants may be prescribed as monotherapy or in combination with biologics, depending on disease severity, patient tolerance, and previous treatment response[3,5,7]. A long-term Dutch study found that over 70% of CD patients received an immunosuppressant within five years of diagnosis[8]. Immunosuppressive monotherapy is often used to maintain remission in patients who initially responded to corticosteroids or induction therapy, thereby reducing the risk of relapse in moderate to severe CD[9,10]. Moreover, combination therapy with biologic agents, especially anti-tumor necrosis factor (anti-TNF) drugs, has become widely adopted due to its synergistic benefits: It reduces immunogenicity of biologics and enhances long-term clinical outcomes, particularly in patients with severe or refractory CD[11,12].
Among the available immunosuppressants, MTX has demonstrated robust efficacy in CD, supported by key clinical studies, including those by Feagan et al[13], Feagan et al[14], Cassinotti et al[15], and the personalized anti-TNF therapy in CD study (2020)[16]. These studies confirm MTX’s role in both induction and maintenance of remission, particularly in corticosteroid-dependent patients or those intolerant to thiopurines. In Europe, MTX is approved [in subcutaneous (SC) formulation only] for “induction of remission in moderate steroid-dependent CD in adult patients in combination with corticosteroids, and for maintenance of remission as monotherapy in patients who have responded to MTX”[17]. Despite this strong evidence base, MTX remains relatively underutilized in CD, especially when compared to its widespread adoption in rheumatology[9,10,18-20]. In contrast, the use of AZA has become more controversial due to its association with an increased risk of malignancies, prompting a reevaluation of its role in long-term CD therapy[21,22]. The differing rates of MTX use between rheumatology and gastroenterology highlight the need for a more nuanced understanding of MTX’s positioning in CD management. Importantly, while MTX’s clinical benefits are increasingly recognized, there is a notable lack of data regarding its routine use in gastroenterology practice in France. To date, no national survey has systematically examined MTX prescribing patterns, physician attitudes, or barriers to use among French gastroenterologists. Given the growing emphasis on evidence-based practice and the need to align real-world prescribing with current clinical guidelines, such data are crucial for informing clinical decision-making and optimizing patient outcomes[9,10,20].
This study addresses this critical gap by presenting findings of a national practice survey conducted among French gastroenterologists. By gathering insights into clinical practices, prescribing patterns, and attitudes toward MTX use, this survey offers a comprehensive overview of real-world treatment patterns, highlights variations in care, and identifies potential gaps between clinical practice and guideline recommendations in France.
The practice survey (questionnaire) was developed by a group of experts from the CREGG (French national association of gastroenterologists, which includes over 1000 gastroenterologists), all of whom have substantial experience in managing CD and the clinical use of MTX. To inform the content and structure of the questionnaire, the experts conducted a targeted review of the scientific literature using PubMed/MEDLINE databases as well as grey literature from clinical guidelines, professional society statements, and patient advocacy resources. This process ensured that the topics addressed in the questionnaire were consistent with current clinical practice and known challenges in MTX use for CD in France. The initial draft of the questionnaire underwent iterative review by the experts to ensure content validity and clarity, and was approved by the CREGG scientific committee prior to its distribution. Although no formal pilot testing with external participants was conducted, the questionnaire was internally pre-tested by the expert group for comprehensibility, relevance, and logical flow. To minimize response bias, the survey was distributed anonymously, and participation was voluntary, without any form of incentive or compensation. Additionally, the survey was designed to avoid leading questions, and response options were structured to capture a broad range of practice patterns without implying preferred answers. Gastroenterologists were invited to participate through CREGG’s newsletter and direct email communication. The final 20-item questionnaire (see Supplementary material) was made available online through a secure platform between March and August 2023. In the survey, disease severity was categorized into three predefined levels, mild, moderate, and severe, based on clinical and biological parameters commonly used in practice: CRP levels were consistently elevated. Prior to accessing the survey, each physician was required to confirm their consent to participate. All responses were collected anonymously and analyzed in aggregate in September 2023. Descriptive statistics were used to summarize the findings.
All gastroenterologists managing patients with CD in France were eligible to participate.
According to French regulations, surveys involving physicians do not require ethics committee approval, as this study does not fall under deliberation No. 2018-154 of May 3, 2018 (JORF No. 0160 of the 13th of July 2018). Data was collected in compliance with the general data protection regulation Europea 2016/679 of April 27, 2016, ensuring anonymity and analysis only in aggregate form. Results are reported descriptively. Personal data (email addresses) were retained only for the survey duration and then deleted.
Out of 116 gastroenterologists who agreed to participate in the survey, 87 completed the questionnaire and met the eligibility criteria (i.e., currently managing CD patients in France). Participation in all questions was optional. While most respondents completed the survey, two did not answer the question regarding MTX prescription status. Prescription data are therefore based on responses from 85 physicians.
The mean age of participants was 50 years (range: 28-71). Respondents were from across France, including major urban centers. Of the participants, 60% (n = 52) worked exclusively in private practice, 26% (n = 23) worked in hospitals (university or general), and 14% (n = 12) in both settings.
A majority of respondents (72%, n = 63) had more than 10 years of experience managing CD patients, while 21% (n = 18) had between 5 and 10 years, and 7% (n = 6) had less than five years. Hospital-based gastroenterologists were generally younger (mean age: 44) compared to those in private practice (mean age: 52). Active caseloads varied significantly, ranging from 7 to about 1500 CD patients per year (mean: 140, median: 50). Hospital-based gastroenterologists reported the highest caseloads (mean: 338, median: 150), compared to private practitioners (mean: 61, median: 30) and those in mixed practices (mean: 140, median: 50). Private practitioners were more likely to treat patients with mild-to-moderate CD (69% of their caseload) compared to hospital-based gastroenterologists (31%) and those in mixed practice (both activity: Private and hospital practice) (50%).
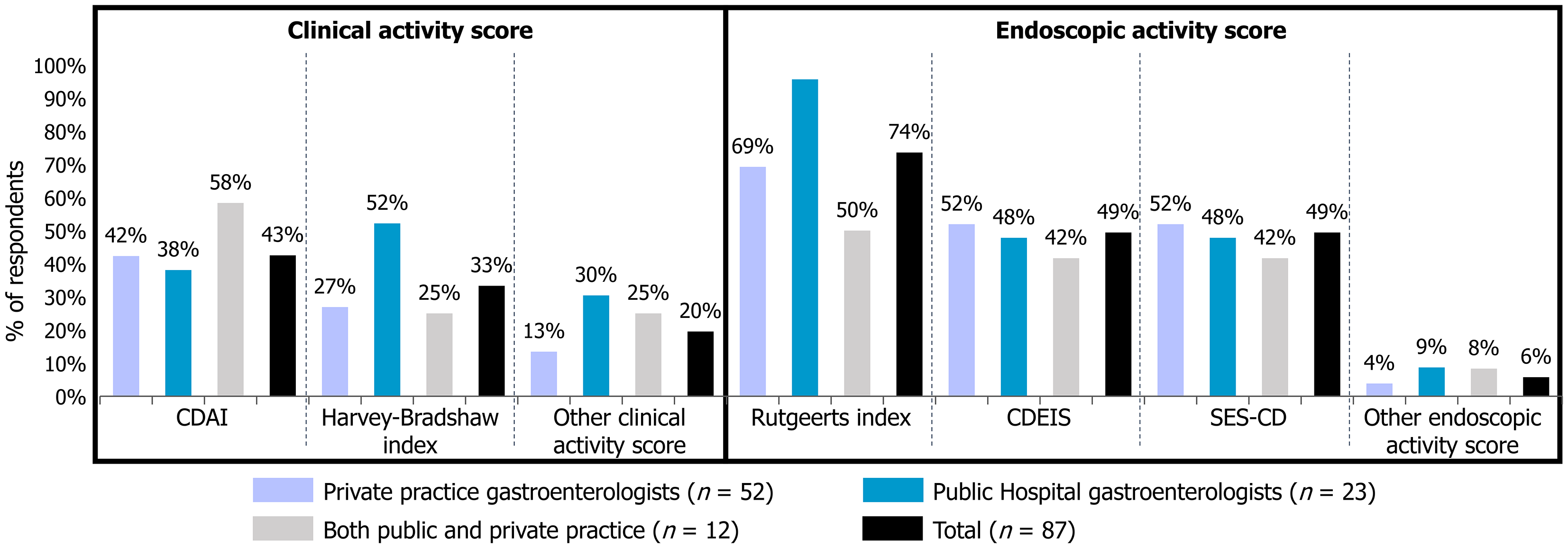
Clinical and endoscopic disease activity scores were used similarly across all groups (Figure 1). The CD activity index (CDAI) was the most commonly used clinical score (43% of respondents), followed by the Harvey-Bradshaw index (33%). For endoscopic scores, the Rutgeerts score was the most used (74%), followed by the CD endoscopic index and the SES-CD (49% each). Hospital-based gastroenterologists mostly used the Harvey-Bradshaw index (52%) and the Rutgeerts score (96%). Overall, the surveyed gastroenterologists reported using a single clinical disease activity score but several endoscopic disease activity scores.
Regarding the pharmacological treatment of CD, the majority of surveyed gastroenterologists (69% of private practitioners and 100% of hospital-based practitioners) followed ECCO and French guidelines. Fewer than 17% reported referring to the American Gastroenterological Association guidelines.
Seventy percent of respondents indicated they consulted a colleague for a second opinion before initiating treatment in at least some cases (approximately 10% of cases on average). In contrast, 30% reported never seeking a colleague’s input. This latter rate (likely not including staff involved in collegial procedures) was higher among hospital-based gastroenterologists (43%) compared to those in private practice (29%) or those in mixed practice setting (17%).
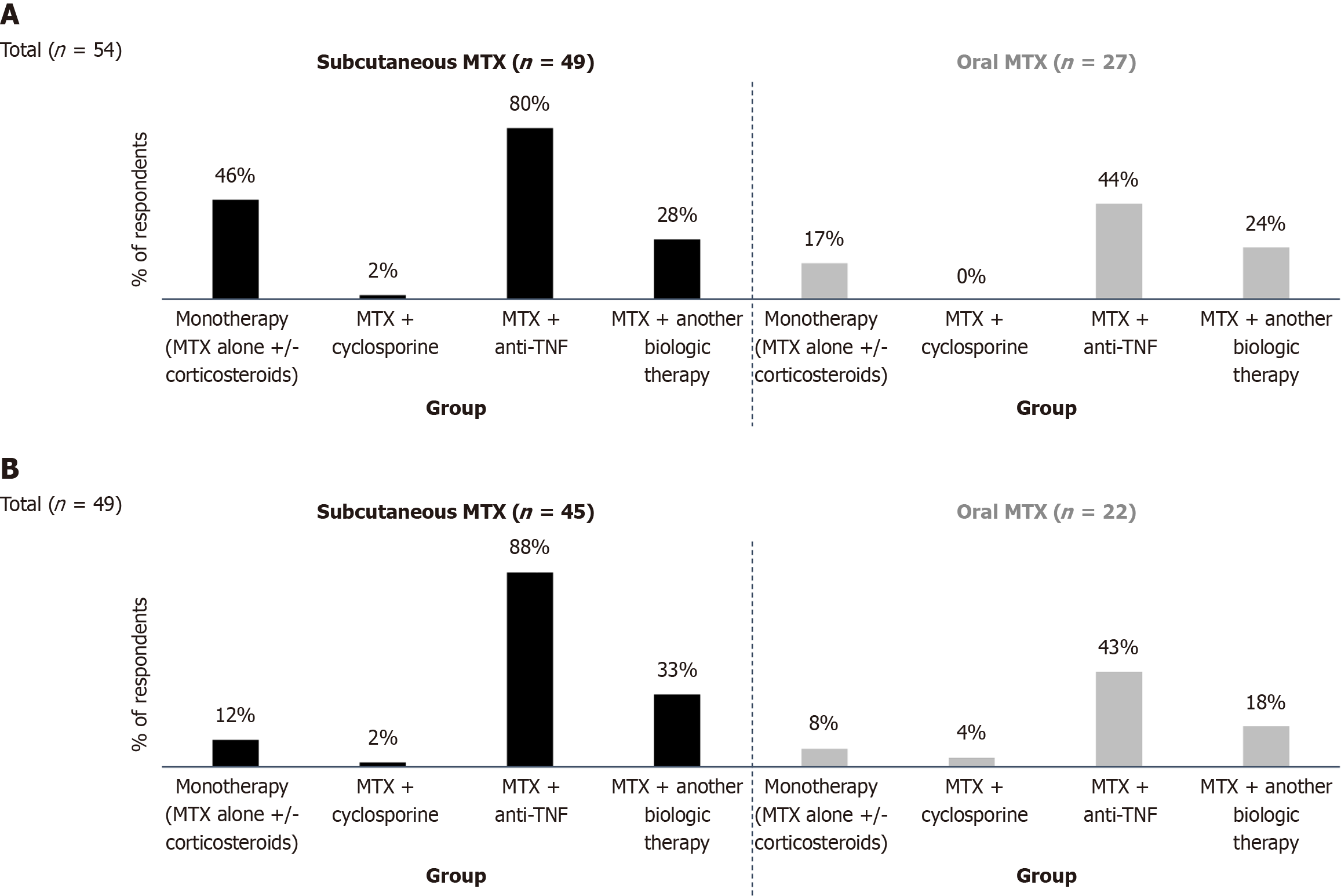
Overall, 71% (n = 60) of respondents reported prescribing MTX, with 52% using only the SC formulation, 8% using only the oral form, and 40% prescribing both. In cases of mild-to-moderate CD, MTX prescribers often combined MTX with a biologic agent: 80% used SC MTX, and 44% used oral MTX in combination with an anti-TNF agent (Figure 2A). Additionally, 46% of respondents prescribed SC MTX either alone or in combination with corticosteroids, while combining MTX with cyclosporine was rare. In severe CD cases, these trends were more pronounced. Among 49 respondents, 88% combined SC MTX and 43% combined oral MTX with an anti-TNF agent, while 33% combined SC MTX and 18% combined oral MTX with another biologic. Only 6% (n = 3) reported using MTX in combination with cyclosporine (Figure 2B).
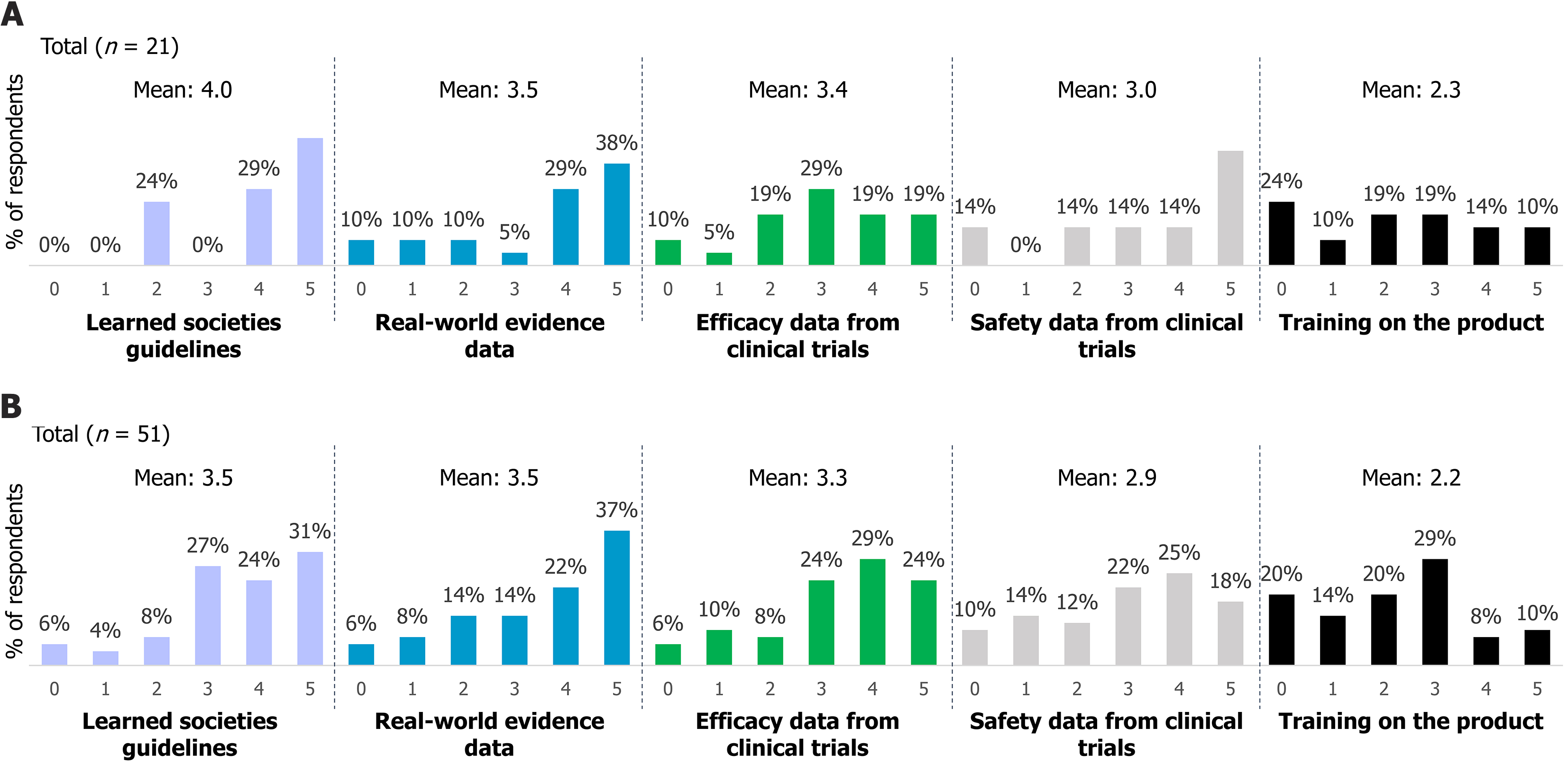
Among the survey respondents, 29% (n = 25) did not prescribe MTX. The main reason cited was habit (60%), followed by a preference for other drugs (36%), limited knowledge of MTX (28%), concerns about safety (12%), and a perceived lack of efficacy (8%). Most non-prescribers indicated that guidelines from learned societies and clinical safety data would encourage them to consider MTX (Figure 3). On a scale from 0 (not useful) to 5 (essential), 77% (16 out of 21 respondents) rated guidelines from learned societies as important (score of 4) or essential (score of 5). Similarly, 67% considered real-world data as important or essential, followed by 57% for clinical safety data, 38% for clinical effectiveness data, and 14% for training on the drug.
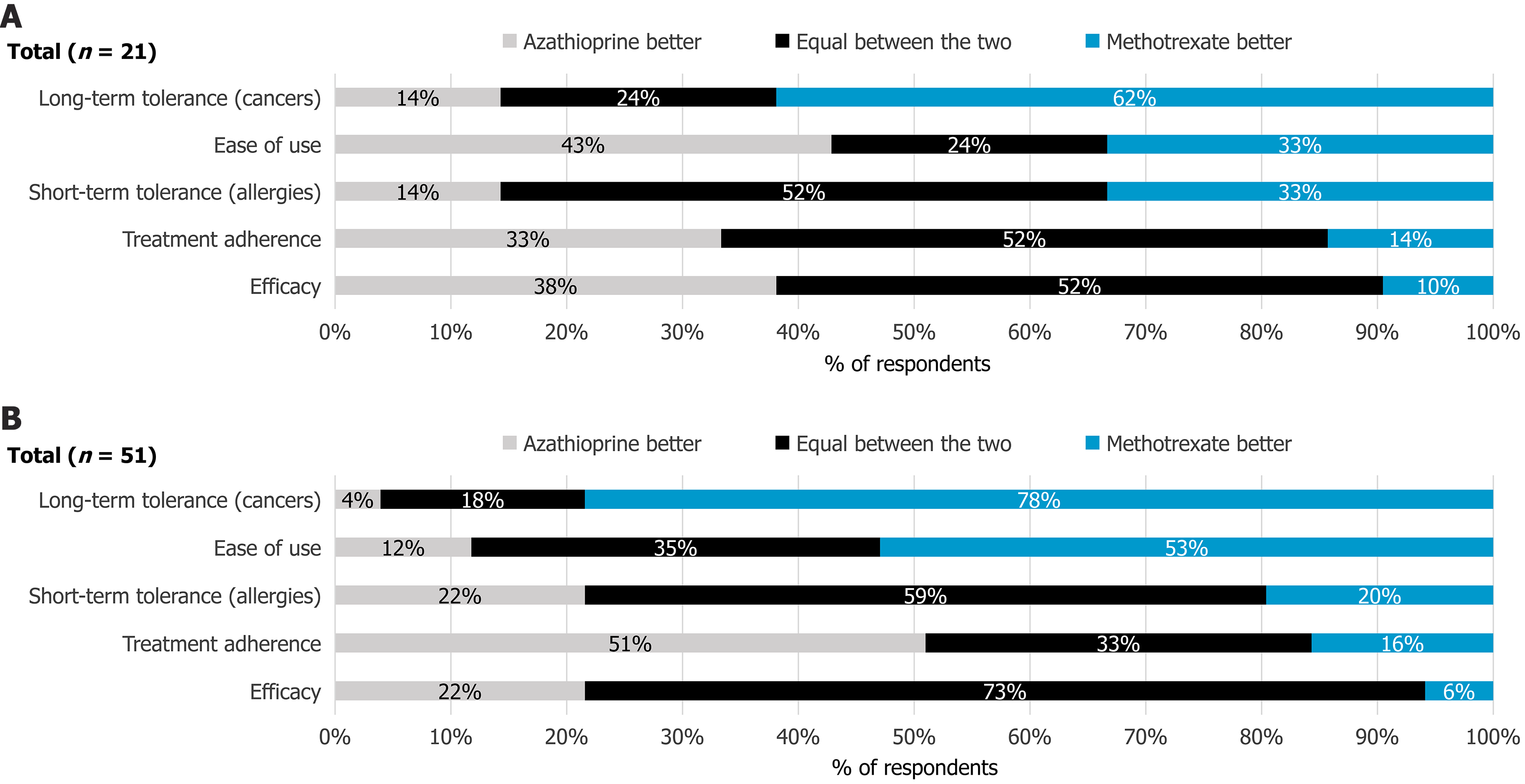
Among MTX non-prescribers, the predominant view was that MTX had a better long-term safety profile than AZA, particularly concerning carcinogenic risk (62%). Both drugs were considered equivalent in terms of effectiveness (52%), short-term tolerability (e.g., allergic reactions 52%), and patient compliance (52%). However, AZA was perceived easier to use than MTX (43%) (Figure 4). Overall, 70% of respondents indicated that concerns about AZA’s long-term safety profile led them to consider MTX as an alternative for CD treatment. Nearly half (48%, n = 11) of MTX non-prescribers believed that prescribing practices for AZA and MTX had shifted since 2019, with 82% observing an increased use of MTX over AZA.
Among MTX prescribers (n = 60), 73% reported using SC MTX for 6 to 24 months in combination therapy to optimize clinical and endoscopic outcomes, while 47% stated prescribing it for more than 24 months. SC MTX was used as monotherapy for 3 to 24 months by 18% of prescribers, and for over 24 months by 45%.
Regarding the choice of MTX formulation, oral MTX prescribers (n = 51) (off-label use) cited patient preference as the primary reason (53%), followed by perceived better compliance (39%), ease of use (37%), a perceived better safety profile (37%), habit (8%), and perceived greater effectiveness (4%).
When combining an immunosuppressant with a biologic or cyclosporine, the most common reason for preferring SC MTX were joint involvement (77%), negative Epstein-Barr virus serological status (65%), age over 65 years (58%), history of cancer (49%), skin manifestations (30%), and other reasons (9%, primarily poor tolerance to mercaptopurine or AZA).
Similar to MTX non-prescribers, most SC MTX prescribers (59%) considered guidelines from learned societies important when evaluating MTX for medically justified cases (Figure 3). However, MTX prescribers placed greater value on real-world data (55%) compared to non-prescribers. Training on MTX use was not considered a key factor in their decision-making.
Among MTX prescribers, 73% considered MTX and AZA equally effective. A majority (59%) also perceived no significant difference in patient compliance. MTX was viewed as safer than AZA in both the short term (allergic reactions 53%) and long term (carcinogenic risk 78%). Conversely, AZA was regarded as easier to use (51%) (Figure 4).
More broadly, 89% of MTX prescribers stated that concerns regarding AZA’s long-term safety influenced their decision to consider MTX for CD treatment a higher proportion than among non-prescribers. Additionally, 64% of MTX prescribers reported that prescribing practices for MTX and AZA had shifted since 2019, with 84% observing a trend toward increased MTX use.
The findings from this survey provide valuable insights into current MTX prescribing practices for CD among French gastroenterologists. The respondent pool was diverse in terms of professional background and experience, with 72% having more than 10 years of clinical practice. This high level of experience enhances the validity of the data, offering a credible reflection of established practice patterns. However, differences in caseload and practice setting, particularly between hospital-based and private practitioners, may influence treatment approaches. The use of standardized tools for assessing disease activity was common. Despite its complexity and reliance on patient dairies and subjective symptoms, the CDAI was used by 43% of respondents[23-25]. The Rutgeerts score, originally developed for postoperative assessment, was used by 74% overall, and notably by 96% of hospital-based gastroenterologists, likely due to its ease of use[26]. While widely applied, the Rutgeerts score does not replace comprehensive indices like the CDEIS or SES-CD in evaluating endoscopic disease activity. It is noteworthy that the Rutgeerts score, although originally validated for assessing postoperative recurrence after ileocolonic resection, was frequently reported by respondents for use in general endoscopic activity evaluation. This mis-application is common in clinical practice but should be interpreted with caution given its original intended use[27].
Adherence to European and national guidelines (ECCO, CREGG) was high, reflecting alignment with evidence-based recommendations. Only 17% of respondents referenced American guidelines, highlighting the regional specificity of CD treatment paradigms.
In terms of MTX prescribing, 71% of respondents reported using the drug, 52% exclusively via the SC route, 8% via the oral route, and 40% using both. This aligns with the superior bioavailability and efficacy of SC MTX and its authorization in CD management[28]. However, the off-label use of oral MTX, while often preferred by patients for its convenience, warrants closer examination. European guidelines, including ECCO (2024)[4], NICE (2019)[16], and CREGG (2021)[29-32], do not recommend the oral route due to variable absorption and reduced efficacy.
The survey identified several clinical profiles in which MTX is commonly prescribed in combination with anti-TNF agents: Patients with articular manifestations (77%), Epstein-Barr virus-negative status (65%), and those over age 65 years (58%). These indications reflect the drug’s well-established role in rheumatoid arthritis[18,19] and the immunosuppressive benefits when co-administered with biologics in populations at higher risk for lymphoproliferative complications[33,34]. ECCO, NICE, and CREGG guidelines support SC MTX, often with corticosteroids or budesonide, for inducing remission in AZA-intolerant patients or those with thiopurine methyltransferase deficiency[4,16,29-32]. The recent GETAID guidelines (2024) also endorse combining MTX with biologics to reduce immunogenicity[35].
International surveys offer comparative insight. In Canada, 33.3% of gastroenterologists reported never prescribing MTX, and only 41.5% used the intramuscular route[36]. In the United Kingdom, MTX use was limited and mainly reserved for co-treatment with biologics to reduce immunogenicity[37]. The MICI-METHO French multicenter study (2025) reported SC administration in 57.8% and oral MTX in 38% of IBD patients, with persistence influenced by gender and extraintestinal symptoms[38]. These findings align with our current survey and underscore the variability in prescribing behavior across countries.
The divergence between MTX prescribers and non-prescribers in our survey was notable. Among the 29% (n = 25) of respondents who did not prescribe MTX, the most frequently cited reason was habit (60%), followed by preference for other drugs (36%), limited knowledge of MTX (28%), safety concerns (12%), and perceived lack of efficacy (8%). Most non-prescribers also indicated that guidelines from learned societies (77%) and real-world data (67%) would encourage them to consider MTX use. While these findings are based on descriptive data, they offer meaningful insight into behavioral barriers and educational gaps. We acknowledge that no statistical comparisons or multivariate analyses were performed to further validate these associations. This limitation is due to the exploratory nature of the study and the lack of statistical power to detect differences between subgroups. Nonetheless, the observed trends remain relevant and provide direction for future hypothesis-driven research with larger samples. Both prescribers and non-prescribers perceived MTX to have a superior long-term safety profile compared to AZA, supported by recent studies showing lower rates of lymphoma and leukopenia[20,39-42]. Still, the belief that MTX is more difficult to use persists, especially among prescribers preferring oral formulations for ease despite efficacy concerns, a pattern also observed among the 37% of oral MTX prescribers.
Several factors may help explain why MTX remains underused in clinical practice despite its favorable long-term safety profile. First, historical prescribing habits in France and elsewhere have long favored thiopurines, especially AZA, which were traditionally considered first-line immunosuppressants in CD. This entrenched practice may have created a degree of therapeutic inertia, especially among clinicians less familiar with MTX’s evolving evidence base. Furthermore, MTX has often been viewed as a “second-line” or “rescue” therapy in gastroenterology rather than a standard maintenance option. This perception may continue to influence its limited uptake. In parallel, the observed off-label use of oral MTX raises important concerns. While oral administration is often preferred by patients for convenience, it is associated with variable absorption and reduced bioavailability, particularly at higher doses, compared to the SC route recommended by guidelines[4,16,29-32]. This discrepancy between guideline recommendations and real-world practice highlights a need for targeted education to ensure that the advantages of SC MTX, both in terms of efficacy and safety, are better communicated to clinicians and patients. Addressing these cultural, historical, and practical barriers may help optimize MTX utilization and align practice more closely with current evidence. Since 2019, there has been a growing trend towards increased MTX use in CD, reflecting enhanced clinician confidence. Recent evidence continues to validate MTX’s effectiveness for steroid-refractory CD and its synergistic role with anti-TNF agents in preventing immunogenicity[43].
Despite the high baseline knowledge among respondents, many expressed the need for additional training and guidance, particularly concerning MTX initiation protocols, safety monitoring, and off-label use. This educational gap presents an opportunity to provide updated, practice-oriented recommendations.
The survey was distributed through French professional networks by the CREGG, ensuring broad coverage of the target audience and the inclusion of gastroenterologists from diverse practice settings, geographical regions, and levels of experience. While a limitation of this study is the relatively modest number of respondents (n = 87), this figure is consistent with typical response rates observed in non-incentivized surveys targeting specialist medical communities. For context, another French nationwide survey on IBD management included 65 private gastroenterologists, further supporting the feasibility and relevance of studies with similar sample sizes in this field[44]. Importantly, this limitation is mitigated by the high level of expertise among participants. The majority were experienced gastroenterologists, with over 10 years of clinical practice and direct involvement in CD management, including MTX use. This expert profile reinforces the clinical relevance, reliability, and practical value of the insights gathered, despite the limited number of respondents. As of the most recent data, approximately 3844 gastroenterologists are practicing in France[45]. However, there are no specific data indicating how many are actively involved in CD care and MTX management. While representativeness remains a limitation, the perspectives captured reflect real-world practices from senior clinicians in the field, particularly valuable in the absence of large-scale national data on MTX use. Moreover, as this was a self-reported, anonymous survey, the data are subject to potential recall and selection bias, notably the possible underrepresentation of non-prescribers. These methodological limitations have been acknowledged and are inherent to cross-sectional survey designs of this nature. Another limitation is the absence of statistical comparisons between subgroups of respondents (e.g., MTX prescribers vs non-prescribers). As the objective of the study was primarily exploratory and descriptive in nature, no statistical power calculations were conducted, and multivariate analyses were not performed. The study was not designed to detect statistically significant differences or establish causal relationships. Instead, it aimed to document real-world practices and identify practice trends and educational gaps, which can inform future, hypothesis-driven research.
To the best of our knowledge, this survey represents the first study on the MTX use in CD management among French gastroenterologists.
In conclusion, this survey highlights the evolving role of MTX in the treatment of CD in France, challenging misconceptions about its safety and reinforcing its validity as a therapeutic option. The choice between the immunosuppressant such as AZA and MTX in CD treatment appears to be influenced by prescribing habit and unfamiliarity with MTX as a treatment option for this disease. The gastroenterologists who participated in this survey had no preconceived ideas about MTX and generally expressed a positive view on this historical product long-term safety profile. However, there remains room for improvement in their knowledge of MTX, particularly regarding best practices. The favorable safety profile of MTX compared to AZA, balanced against the greater ease of use associated with the oral formulation, should encourage increased utilization of MTX. The recently published French GETAID guidelines (2024) clearly position immunosuppressants, particularly MTX, as part of combination strategies with biologics to prevent immunogenicity. It will be interesting to observe how prescribing patterns and the role of MTX in CD management evolve in response to these new guidelines.
The authors express their gratitude to all the physicians who participated in the survey. Special thanks go to Goff YL and Clanche SL from Public Health Expertise for their contributions to survey analysis, medical writing, and editorial support.
| 1. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4467] [Article Influence: 496.3] [Reference Citation Analysis (111)] |
| 2. | Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S, Deardon R, Jones JL, Kuenzig ME, Leddin D, McBrien KA, Murthy SK, Nguyen GC, Otley AR, Panaccione R, Rezaie A, Rosenfeld G, Peña-Sánchez JN, Singh H, Targownik LE, Kaplan GG. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology. 2019;156:1345-1353.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 351] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 3. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1950] [Article Influence: 390.0] [Reference Citation Analysis (1)] |
| 4. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 187] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 5. | NICE. Crohn’s disease: management. [cited July 21, 2025]. Available from: https://www.nice.org.uk/guidance/ng129/chapter/Recommendations. |
| 6. | Sriranganathan D, Segal JP, Garg M. Biologics recommendations in the ECCO guidelines on therapeutics in Crohn's disease: medical treatment. Frontline Gastroenterol. 2022;13:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 978] [Article Influence: 163.0] [Reference Citation Analysis (2)] |
| 8. | Jeuring SF, van den Heuvel TR, Liu LY, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, Masclee AA, Jonkers DM, Pierik MJ. Improvements in the Long-Term Outcome of Crohn's Disease Over the Past Two Decades and the Relation to Changes in Medical Management: Results from the Population-Based IBDSL Cohort. Am J Gastroenterol. 2017;112:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Nancey S, Hagège H. Use of methotrexate in Crohn’s disease. Hépato-Gastro Oncologie Digestive. 2024;31:15-19. [DOI] [Full Text] |
| 10. | Nancey S, Laharie D. Methotrexate and IBD: mechanisms of action, indications/use. Hépato-Gastro Oncologie Digestive. 2024;31:8-14. [DOI] [Full Text] |
| 11. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2451] [Article Influence: 153.2] [Reference Citation Analysis (1)] |
| 12. | Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY, Kaplan GG. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. 2015;148:344-54.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med. 1995;332:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 615] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M, Hanauer SB, McDonald JW. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 519] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Cassinotti A, Batticciotto A, Parravicini M, Lombardo M, Radice P, Cortelezzi CC, Segato S, Zanzi F, Cappelli A, Segato S. Evidence-based efficacy of methotrexate in adult Crohn's disease in different intestinal and extraintestinal indications. Therap Adv Gastroenterol. 2022;15:17562848221085889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 517] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 17. | European Medicines Agency. Nordimet. [cited July 21, 2025]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/nordimet. |
| 18. | Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol. 2020;16:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 19. | Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 289] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Goëb V, Hagège H. Methotrexate tolerance: myths and realities. Hépato-Gastro Oncologie Digestive. 2024;31:20-28. [DOI] [Full Text] |
| 21. | Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Laredo V, García-Mateo S, Martínez-Domínguez SJ, López de la Cruz J, Gargallo-Puyuelo CJ, Gomollón F. Risk of Cancer in Patients with Inflammatory Bowel Diseases and Keys for Patient Management. Cancers (Basel). 2023;15:871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 23. | Thia KT, Sandborn WJ, Lewis JD, Loftus EV Jr, Feagan BG, Steinhart AH, Hanauer SB, Persson T, Sands BE. Defining the optimal response criteria for the Crohn's disease activity index for induction studies in patients with mildly to moderately active Crohn's disease. Am J Gastroenterol. 2008;103:3123-3131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Sands BE, Ooi CJ. A survey of methodological variation in the Crohn's disease activity index. Inflamm Bowel Dis. 2005;11:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | de Dombal FT, Softley A. IOIBD report no 1: Observer variation in calculating indices of severity and activity in Crohn's disease. International Organisation for the Study of Inflammatory Bowel Disease. Gut. 1987;28:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1272] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 27. | Pal P, Reddy DN, Rao GV. Endoscopic Assessment of Postoperative Recurrence in Crohn's Disease: Evolving Concepts. Gastrointest Endosc Clin N Am. 2025;35:121-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Bujor AM, Janjua S, LaValley MP, Duran J, Braun J, Felson DT. Comparison of oral versus parenteral methotrexate in the treatment of rheumatoid arthritis: A meta-analysis. PLoS One. 2019;14:e0221823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Amiot A, Bouguen G, Bonnaud G, Bouhnik Y, Hagege H, Peyrin-Biroulet L; French National Consensus Clinical guidelines for the management of IBD study group. Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig Liver Dis. 2021;53:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Carbonnel F, Colombel JF, Filippi J, Katsanos KH, Peyrin-Biroulet L, Allez M, Nachury M, Novacek G, Danese S, Abitbol V, Bossa F, Moreau J, Bommelaer G, Bourreille A, Fumery M, Roblin X, Reinisch W, Bouhnik Y, Brixi H, Seksik P, Malamut G, Färkkilä M, Coulibaly B, Dewit O, Louis E, Deplanque D, Michetti P, Sarter H, Laharie D; European Crohn's and Colitis Organisation; Groupe d'Étude Thérapeutique des Affections Inflammatoires Digestives. Methotrexate Is Not Superior to Placebo for Inducing Steroid-Free Remission, but Induces Steroid-Free Clinical Remission in a Larger Proportion of Patients With Ulcerative Colitis. Gastroenterology. 2016;150:380-8.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | CREGG. Comment Prescrire les Traitements des MICI. [cited July 21, 2025]. Available from: https://www.cregg.org/commissions/mici/outils-mici/comment-prescrire-les-traitements-des-mici/. |
| 32. | CREGG. Méthotrexate. [cited July 21, 2025]. Available from: https://www.cregg.org/commissions/mici/outils-mici/comment-prescrire-les-traitements-des-mici/methotrexate/. |
| 33. | Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM, Higgins P, Katsanos KH, Nissen L, Pellino G, Rogler G, Scaldaferri F, Szymanska E, Eliakim R; ECCO. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 34. | Gabbani T, Deiana S, Lunardi S, Manetti N, Annese V. Safety profile of methotrexate in inflammatory bowel disease. Expert Opin Drug Saf. 2016;15:1427-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Bouguen G, Gornet J, Hamoudi N, Geyl S, Bresteau C, Buisson A, Le Cosquer G, Caron B, Hupe M, Fumery M, Charkaoui M, Richard N, Martin A, Juillierat P, Le Berre C, Biron A, Wils P, Faure P, Heluwaert F, Paupart T, Abitbol V, Vuitton L, Amiot A. French recommendations for the management of Crohn’s disease – Short version. Hépato-Gastro Oncologie Digestive. 2024;31:871-900. [DOI] [Full Text] |
| 36. | Chande N, Ponich T, Gregor J. A survey of Canadian gastroenterologists about the use of methotrexate in patients with Crohn's disease. Can J Gastroenterol. 2005;19:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Wang M, Zhao J, Wang H, Zheng C, Chang B, Sang L. Methotrexate showed efficacy both in Crohn's disease and ulcerative colitis, predictors of surgery were identified in patients initially treated with methotrexate monotherapy. Front Pharmacol. 2022;13:996065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 38. | Caron B, Kirchgesner J, Nachury M, Bouguen G, Uzzan M, Buisson A, Le Cosquer G, Vuitton L, Seksik P, Petit S, Wils P, Nancey S, Altwegg R, Hupe M, Fumery M, Roblin X, Laharie D, Hebuterne X, Baumann C, Vicaut E, Peyrin-biroulet L. P0704 A Multicenter Study Evaluating the Persistence, Effectiveness, and Safety of Methotrexate in Patients with Inflammatory Bowel Disease: The MICI-METHO Study. J Crohn’s Colitis. 2025;19:i1375-i1375. [DOI] [Full Text] |
| 39. | Atia O, Friss C, Ledderman N, Greenfeld S, Kariv R, Daher S, Yanai H, Loewenberg Weisband Y, Matz E, Dotan I, Turner D. Thiopurines Have Longer Treatment Durability than Methotrexate in Adults and Children with Crohn's Disease: A Nationwide Analysis from the epi-IIRN Cohort. J Crohns Colitis. 2023;17:1614-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, Bombardier C, Wells GA, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;2013:CD000951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 41. | Nakafero G, Grainge MJ, Williams HC, Card T, Taal MW, Aithal GP, Fox CP, Mallen CD, van der Windt DA, Stevenson MD, Riley RD, Abhishek A. Risk stratified monitoring for methotrexate toxicity in immune mediated inflammatory diseases: prognostic model development and validation using primary care data from the UK. BMJ. 2023;381:e074678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 42. | Solomon DH, Glynn RJ, Karlson EW, Lu F, Corrigan C, Colls J, Xu C, MacFadyen J, Barbhaiya M, Berliner N, Dellaripa PF, Everett BM, Pradhan AD, Hammond SP, Murray M, Rao DA, Ritter SY, Rutherford A, Sparks JA, Stratton J, Suh DH, Tedeschi SK, Vanni KMM, Paynter NP, Ridker PM. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann Intern Med. 2020;172:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 43. | Sequier L, Caron B, Loeuille D, Honap S, Jairath V, Netter P, Danese S, Sibilia J, Peyrin-Biroulet L. Systematic review: Methotrexate-A poorly understood and underused medication in inflammatory bowel disease. Aliment Pharmacol Ther. 2024;60:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 44. | Duchesne C, Faure P, Kohler F, Pingannaud MP, Bonnaud G, Devulder F, Abramowitz L, Boustière C, Peyrin-Biroulet L; CREGG (Club de Reflexion des cabinets et Groupes d’Hépato-Gastroentérologie). Management of inflammatory bowel disease in France: a nationwide survey among private gastroenterologists. Dig Liver Dis. 2014;46:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Profilmedecin. Chiffres clés : Gastro-entérologue. [cited July 21, 2025]. Available from: https://www.profilmedecin.fr/contenu/chiffres-cles-medecin-gastro-enterologue/. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/